| CATEGORII DOCUMENTE |
| Alimentatie nutritie | Asistenta sociala | Cosmetica frumusete | Logopedie | Retete culinare | Sport |
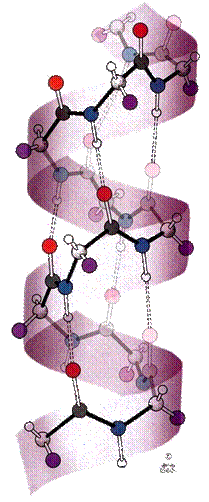 Proteinele
sunt componente de baza ale tuturor celulelor vii, alaturi de lipide, zaharide,
vitamine, enzime, apa si saruri anorganice, formand impreuna un sistem complex
in cadrul caruia se petrec o serie de reactii chimice care asigura
reproducerea, dezvoltarea si functionarea normala a fiintelor vii.
Proteinele
sunt componente de baza ale tuturor celulelor vii, alaturi de lipide, zaharide,
vitamine, enzime, apa si saruri anorganice, formand impreuna un sistem complex
in cadrul caruia se petrec o serie de reactii chimice care asigura
reproducerea, dezvoltarea si functionarea normala a fiintelor vii.  Proteinele solubile sau globulare apar in celule in
stare dizolvata sau sub forma de geluri hidratate. Ele au insusiri fiziologice
specifice si se subimpart in albumine si globuline.
Proteinele solubile sau globulare apar in celule in
stare dizolvata sau sub forma de geluri hidratate. Ele au insusiri fiziologice
specifice si se subimpart in albumine si globuline.
|
Nr. Crt. |
Formula de structura |
Denumire uzualaPrescurtari IUPAC |
Denumire rationala |
|
|
|
|
|
|
|
Aminoacizi cu radical nepolar |
(hidrofob) |
|
|
|
CH2-COOHNH2 |
GlicinaGly, G |
acid a-aminoacetic |
|
|
CH3-CH-COOHNH2 |
AlaninaAla, A |
acid a-aminopropionic |
|
|
CH3-CH-CH-COOHCH3 NH2 |
ValinaVal, V |
acid a-aminoizovalerianic |
|
|
CH3-CH-CH2-CH-COOHCH3 NH2 |
LeucinaLeu, L |
Acid a-aminoizocapronic |
|
|
CH3-CH2-CH-CH-COOHCH3 NH2 |
IzoleucinaIle, I |
Acid a-amino-b-metil valerianic |
|
|
C6H5-CH2-CH-COOHNH2 |
FenilalaninaPhe, F |
Acid b-fenil-a-amino propionic |
|
|
|
|
|
|
|
CH3-S-CH2-CH2-CH-COOHNH2 |
MetioninaMet, M |
Acid a-amino g-metiltiobutiric |
|
|
Aminoacizi cu radical polar, |
neincarcat electric la |
pH |
|
|
HO-CH2-CH-COOHNH2 |
SerinaSer, S |
Acid a-amino b-hidroxipropionic |
|
|
CH3-CH-CH-COOHOH NH2 |
TreoninaThr, T |
Acid a-amino b-hidroxibutiric |
|
|
HS-CH2-CH-COOHNH2 |
CisteinaCys, C |
Acid a-amino b-tiopropionic |
|
|
HO-C6H4-CH2-CH-COOHNH2 |
TirosinaTyr, Y |
p-hidroxifenil alanina |
|
|
H2NOC-CH2-CH-COOHNH2 |
AsparaginaAsn,N |
Acid a-amino b-amidosuccinic |
|
|
H2NOC-CH2-CH2-CH-COOHNH2 |
GlutaminaGln, Q |
Acid a-amino g-amidoglutaric |
|
|
Aminoacizi cu radical polar, incarcat |
negativ la pH |
|
|
|
HOOC-CH2-CH-COOHNH2 |
Acid aspartic (asparagic), Asp,D |
Acid aminosuccinic |
|
|
HOOC-CH2-CH2-CH-COOHNH2 |
Acid glutamicGlu, E |
Acid a-amino glutaric |
|
|
Aminoacizi cu radical polar, incarcat |
pozitiv la pH=6 |
|
|
|
CH2-CH2-CH2-CH2-CH-COOHNH2 NH2 |
LisinaLys,K |
Acid a e-diamino caproic |
|
|
H2N-C-NH-CH2-CH2-CH2-CH-COOHNH NH2 |
ArgininaArg,R |
Acid a-aminod-guanidinovalerianic |
|
|
N C-CH2-CH-COOHCH CH NH2NH |
HistidinaHis, H |
Acid a-amino b-imidazolil propionic |
Nr. crt. |
Formula de structura |
Denumire uzualaPrescurtari IUPAC |
Denumire rationala |
|
|
|
|
|
|
|
H2N-CH2-CH2-COOH |
b-alanina |
Acid b-amino propionic |
|
|
H2N-CH2-CH2-CH2-COOH |
Acid g-aminobutiric |
|
|
|
H2N-CH2-CO-CH2-CH2-COOH |
Acid aminolevulinic |
|
|
|
H2N-CO-NH-(CH2)3-CH-COOHNH2 |
Citrulina |
Acid a-amino d-amidinovalerianic |
|
|
H2N-(CH2)3-CH-COOHNH2 |
Ornitina |
Acid a d-diamino valerianic |
|
|
HS-CH2-CH2-CH-COOHNH2 |
Homocistina |
Acid a-amino g-tiobutiric |
|
|
HO-CH2-CH2-CH-COOHNH2 |
Homoserina |
Acid a-amino g-hidroxibutiric |
|
|
H2N-C6H4-COOH |
Acid p-aminobenzoic |
|
|
|
CH3-NH-CH2-COOH |
Sarcozina |
N-metil glicina |
|
|
(CH3)3N+-CH2-COOH |
Betaina |
|
|
|
H2N-C-NH-O-CH2-CH2-CH-COOHNH NH2 |
Canavanina |
|
|
|
HOOC-CH-(CH2)2-S-CH2-CH-COOHNH2 NH2 |
Acid djencolic |
|
|
|
N C-CH2-CH-COOHNH2 |
b-cianoalanina |
|
Nr.crt. |
Aminoacidul |
Forma dePrezentare |
p.t. |
pHi |
Rotatiaoptica |
MD |
SolubilitateaIn apa la pHi |
|
|
Glicocol |
Monolitic |
233d |
|
|
|
|
|
|
Alanina |
Rombic |
297d |
|
|
|
|
|
|
Valina |
Foite |
|
|
|
|
|
|
|
Leucina |
Foite |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Izoleucina |
Placute |
280d |
|
|
|
|
|
|
Serina |
Placute |
228d |
|
|
|
|
|
|
Treonina |
Cristale |
|
|
|
|
|
|
|
Tirosina |
Ace |
314d |
|
|
|
|
|
|
Fenilalanina |
Foite |
283d |
|
|
|
|
|
|
Triptofan |
Placute |
293d |
|
|
|
|
|
|
Acid aspartic |
Foite rombice |
|
|
|
|
|
|
|
Acid glutamic |
Rombic |
|
|
|
|
|
|
|
Glutamina |
Ace |
|
|
|
|
|
|
|
Asparagina |
Cristale |
|
|
|
|
|
|
|
Lisina |
Ace sau placi |
224d |
|
|
|
f. solubil |
|
|
Arginina |
Foite, prisme |
238d |
|
|
|
f. solubil |
|
|
Histidina |
Foite |
|
|
|
|
|
|
|
Cisteina |
Pulberecristalina |
260 |
|
|
|
f. solubil |
|
|
Metionina |
Placute hexagonal |
|
|
|
|
|
|
|
Cistina |
Placute |
259d |
|
|
|
|
|
|
Prolina |
Ace |
|
|
|
|
|
|
|
Hidroxiprolina |
Placute |
|
|
|
|
|
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 4661
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2025 . All rights reserved