| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
DOCUMENTE SIMILARE |
|
TERMENI importanti pentru acest document |
|
Determination Of A Rate Equation
Aim
My aim is to plan an experimental procedure, which will lead to a graphical method to determine how the concentration or volume of each component affects the rate of reaction of the equation:
![]() 2HCl(aq) + Na S O (aq) 2NaCl(aq) + SO (g) + S(s) + H O(l)
2HCl(aq) + Na S O (aq) 2NaCl(aq) + SO (g) + S(s) + H O(l)
In a demonstration shown by the teacher who mixed together 50cm³ of 0.4 mol dmˉ³ Na S O (aq) with 5.0 cm³ of 0.2 mol dmˉ³ HCl(aq) diluted with 20cm³ of H O. The appearance of a precipitate turned the mixture solution milky yellow. The time taken for the mixture solution to turn completely milky white can be used to calculate the rate of reaction.
Equipment And Apparatus
Independent Variable And Dependent Variable.
The Independent Variable of this experiment is the concentration of one substance under investigation.
The Dependent Variable of this experiment is the rate of reaction.
Controlled Variables
Use Of Tile Marked With The Cross Use the same tile that is marked with the cross for each experiment. This is because if I use a different tile marked with a cross for each experiment may affect my results, as the tiles might have crosses different shades of black. Some crosses can be slightly darker, which could take more time to disappear, where as some crosses can be of a lighter shade, which will take less time to disappear.
Concentration Of Components Not Under Investigation If the concentration of any one chemical not under investigation is increased the rate of reaction will also increase as this is because there are more molecules so they are more likely to collide and react successfully. If the concentration of any one chemical is decreased the rate of reaction will also decrease. I will keep the chemicals not under investigation separate from the reaction and keep lids on top of the chemical containers.
Judgement Of When To Stop The Stopwatch Use the same person to make judgements of when the cross has disappeared, as different people can have different judgements based on when the cross has disappeared, which can affect my results.
Safety And Precautions
Safety for the reaction:
![]() 2HCl(aq) + Na S O (aq) 2NaCl(aq) + SO (g) + S(s) + H O(l)
2HCl(aq) + Na S O (aq) 2NaCl(aq) + SO (g) + S(s) + H O(l)
To prevent any of the chemicals to get into the eye, wear eye protection such as Safety Spectacles. Also to protect the skin, wear Gloves on your hands and wear on a Laboratory Coat.
Hydrochloric Acid (HCL) This is an Irritant substance and produces a irritating vapour. To protect yourself against the irritating vapour use substance in the Fume Cupboards and in a well ventilated room. If HCL gets in contact with the skin and eyes wash thoroughly with plenty of water. Take off contaminated clothing. If split in the laboratory wash area with plenty of water.
Sodium Thiosulphate This is a Harmful chemical if ingested. If ingested drink plenty of water and seek medical advice. If Sodium Thiosulphate gets in contact with the skin and eyes wash thoroughly with plenty of water. Take off contaminated clothing. If split in the laboratory, scoop of as much solid as you can and wash area with plenty of water.
Sulphur Dioxide (SO (g)) Toxic by inhalation. Irritating to the eyes and respiratory system. If inhaled remove victim to fresh air to rest and keep warm. Also seek medical advice. Carry out the reaction in a fume cupboard to prevent the Sulphur Dioxide gas produced from escaping.
Procedure
Varying The 0.2 mol dmˉ³ Hydrochloric Acid, HCl(aq).
|
Table 1 |
|
0.2 mol dmˉ³ Hydrochloric Acid, HCl(aq)/cm³ |
0.4 mol dmˉ³ Sodium Thiosulphate, Na S O (aq)/ cm³ |
Volume Of H O Added To HCl(aq)/cm³ |
Time Taken/Sec |
Rate/Secˉ¹ |
Varying The 0.4 mol dmˉ³ Sodium Thiosulphate, Na S O (aq).
|
Table 2 |
|
0.2 mol dmˉ³ Hydrochloric Acid, HCl(aq)/cm³ |
0.4 mol dmˉ³ Sodium Thiosulphate, Na S O (aq)/cm³ |
Volume Of H O Added To Na S O (aq)/ cm³ |
Time Taken/Sec |
Rate/Secˉ¹ |
To work out the rate equation you have to use the formula:
![]() Rate = 1
Rate = 1
Time
I will write the rate of each volume I vary in my range, in the appropriate tables
Order of reaction of each chemical can be worked out by comparing two experiments from each set by using the formula:
There are three types of orders of reaction, 0 Order, 1st Order and 2nd Order, which can be graphically displayed. The graphs below are the graphs that represent each Order.
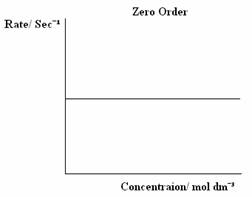
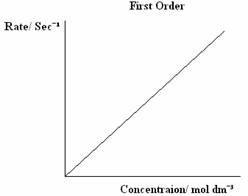
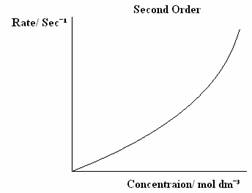
The rate equation is written as:
Zero Order - The rate is constant and is unaffected by changes in concentration.
First Order - The rate doubles as the concentration doubles.
Second Order - The rate increases by four times as the concentration doubles.
You can work out the rate constant by rearranging the formula.
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 1801
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2025 . All rights reserved