| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
Jessica Svensson
9A IESJ
We did this experiment to see the rate of reaction when mixing calcium carbonate with hydrochloric acid under different conditions.
Materials:
Measuring cylinder Conical flask Hot plate Spatula
50 ml 1M hydrochloric acid (HCl) x3 50 ml 2.0M hydrochloric acid x1
Calcium carbonate (chips) Calcium carbonate (powder) Electronic balance
Thermometer
Measure 50 ml of HCl into the conical flask and then add 3g of calcium carbonate. Do this four times and measure the mass every 2 minutes.
In the first experiment, do nothing special. In the second experiment, use powdered calcium carbonate instead of chips. In the third experiment, change the temperature by using the hot plate. In the fourth experiment, use a double concentration of acid.
Make sure to wear safety goggles at all times, as the acid may cause damage to your eyes.
Results:
|
Concentration |
Temp sC |
Size of |
Mass (g) | |||||
|
of acid |
CaCO3 |
0 min |
2 min |
4 min |
6 min |
8 min |
10 min |
|
|
Exp.1 1 |
Normal | |||||||
|
Exp.2 1 |
Powder | |||||||
|
Exp.3 1 |
Normal | |||||||
|
Exp.4 2 |
Normal |
Conclusion
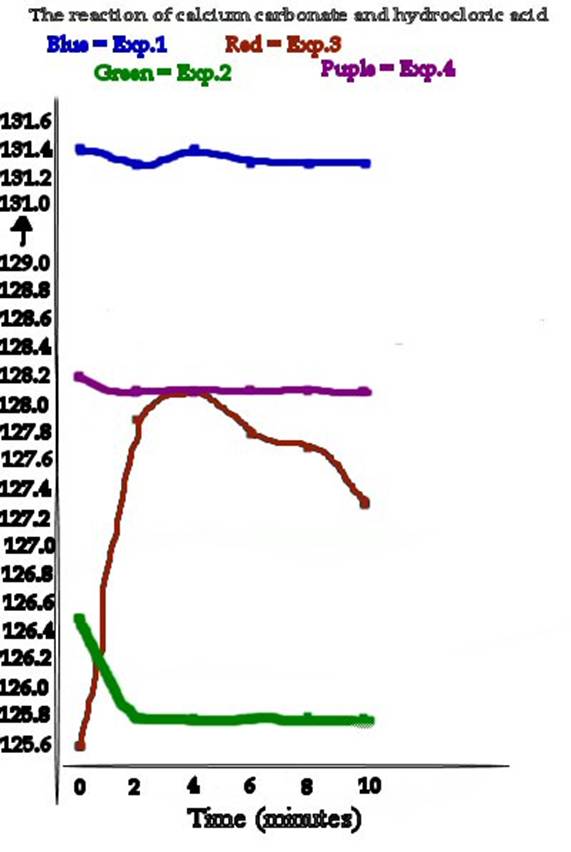
Experiment 2 had the fastest reaction, while the slowest reaction belonged to experiment 3. This shows that changing the temperature didnt help a lot, but the most probable reason this happened is for a fault in the experiment. That experiment 2 was the fastest shows that its easier to react with the acid when you have a powder than with chips. This is because the smaller the particles, the less there is to break down for the acid.
Evaluation
This experiment could have been done a lot better. If you look at the results, some of them show that the experiment gained weight from time to time instead of loosing it.
One thing that could have made this better would have been to make sure all chips were the same size. This would make all the experiments equal from the start, until you change the conditions.
Another would be to have even more accurate scales.
Also, performing these experiments several times instead of just once per condition would have helped to get a more accurate result.
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 1933
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2024 . All rights reserved