| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
CELL INJURY, DYSFUNCTION, AND ADAPTATION
Contents
1. DEFINITION OF DISEASE
Disease is the inability to maintain homeostasis and/or full function
Homeostasis represents the functional and structural equilibrium of cells order to maintain the normal function of entire human organism; requires functional cooperation of widely distributed cells.
Diseases classification:
etiologic classification: traumatic disease, infectious diseases, immune diseases, metabolic diseases.
semiologic classification: acute diseases, chronic disease
anatomic classification: heart diseases, cerebral diseases, hepatic diseases, lung diseases, kidney diseases etc.
ontogenic classification: hereditary diseases, congenital disease, acquired diseases.
2. ETIOLOGIC FACTORS
Etiologic factors are the initiating agents in cell injury and dysfunction.
Etiologic factors classification
A. Concerning to pathogenesis:
determinant etiologic factors : physical, chemical, biological agents;
secondary factors: malnutrition, cold, concomitant chronic diseases, iatrogenic factors.
B. Concerning the origin:
- endogenous factors: hypoxia/ischemia may lead to myocardial infarction, stroke etc ; chemically reactive molecules (free radicals, oxidants) may lead to cells injury, immune effectors proteins and cells lead to immune inflammation, growth factor stimulation or removal may lead to neovascularisation, neoplasia or wound repair disorders ; atherosclerosis lead to myocardial infarction, stroke, peripheral arterial disease ; diabetes mellitus lead to diabetic neuropathy, diabetic retinopathy, diabetic nephropathy
- exogenous factors: physical, chemical, biological, stressing factors.
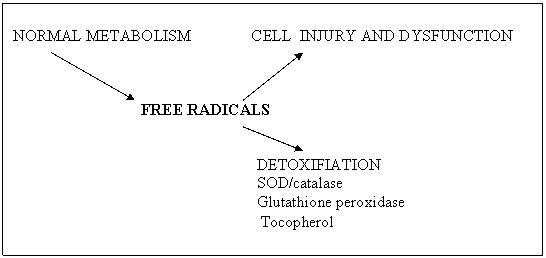
Fig 1. Free radicals action
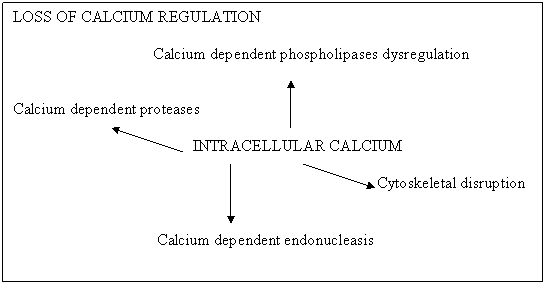
Fig.2. Mechanism of intracellular dysfunction (calcium dysregulation).
3. PATHOGENESIS
Pathogenesis refers to mechanisms of diseases from etiology to complications
Mechanisms of disease
lethal cytotoxicity in critical cell populations with loss of cell function and associate inflammation;
reversible injury to critical cell populations with decreased cells functions.
disruption of cell-cell communication without direct injury.
Classification of pathogenetic mechanisms according to the level of disturbances:
molecular pathogenetic mechanism;
cellular pahogenetic mechanism;
organ pathogenetic mechanism;
systemic pathogenetic mechanism;
integrative pathogenetic mechanism (neuro-endocrine integrative mechanism) (fig.3).
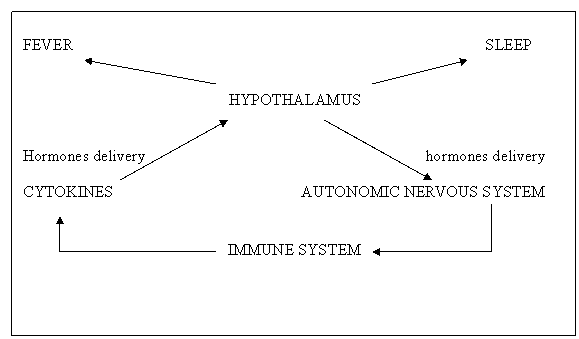
Fig.3. Neuroendocrine integrative pathogenetic mechanism.
4. RISK FACTORS
Risk factors represent the conditions who refers to transition to healthy organism to disease.
Classification of risk factors
Intrinsic risk factors:
inherited risk factors increase the risk for certain diseases like multiple sclerosis in Nordic populations, Behcet disease in Turkish population, sickle anemia in African and Afro-American population.
constitutional diseases increase of sympathetic activity increase the risk for arterial hypertension.
Extrinsic risk factors:
ambient risk factors physical conditions (temperature, pressure, ionizing radiations), biological risk factors (bacterial, viral, worms infections, fungal infections);
geographical risk factors areas with increase of risk for malaria and another tropical diseases
psychical risk factors stress.
nutritional risk factors lipids excesses lead to increase the risk for atherosclerosis, inadequate calcium ingestion lead to increase the risk for tetanus.
iatrogenic risk factors - inadequate drug therapy, inadequate surgical interventions.
HOST DEFENCES
The host defense mechanisms consist in:
physical and chemical barriers are a non-specific mechanism of defenses and consist in:
inflammatory response circulating phagocytes, complement, inflammatory mediators
reticuloendothelial system tissue phagocytes: liver Kupffer cells, lung alveolar macrophages, brain microglial cells, spleen, lymph nodes
fever
immune response
Interference with any of these mechanisms can increase the host susceptibility to disease.
ISCHEMIC CELL DYSFUNCTION
Ischemia represents an inadequate tissue blood supply. Loss of normal blood flow and oxygenation in certain tissues lead to reversible and irreversible cell dysfunctions. The basis modifications which may lead to reversible or irreversible cells dysfunction is the ischemic cascade.
The ischemic cascade is a series of biochemical reactions that taken place in aerobic tissues after seconds to minutes of ischemia. This is typically secondary to stroke injury, or myocardial infarction. Most ischemic cells die do so due to the activation of chemical produced during and after ischemia. The ischemic cascade usually goes on for to three hours but can last for days, even after normal blood flow returns. The ischemic cascade can be generally characterized as follows:
1. Lack of oxygen causes the cells normal processes for making ATP for energy to fail. The increased amount of AMP (from un-recycled ATP) stimulates anaerobic glycolysis (AMP stimulate phosphofructokinase), glycogen is depleted, lactic acid (the end product of glycolisis) and phosphorus (from the ATP and other energy rich phosphates) accumulates, and cells pH drops precipitously, denaturing the proteins.
2. ATP-reliant ion transport pumps fail, causing the cell to become depolarized, allowing ions, including calcium, to flow into the cell. The ion pumps can no longer transport calcium into the cell, and intracellular calcium levels get too high.
3. In the neurons, the presence of calcium, triggers the release the excitatory amino acid neurotransmitter glutamate ; glutamate stimulates the AMPA receptors and calcium permeable NMDA receptors, which open to allow more calcium into cells. Calcium can also cause the release of more glutamate. This process is called excitotoxicity.
4. Excess calcium entry causes the generation of harmful chemicals like free radicals, and calcium dependent enzymes such as calpain, endonucleases, ATPases, and phospholipases
5. As the cells membrane is broken down by phospholipases, it becomes more permeable, and more ions and harmful chemicals flow into the cell.
6. The caspase-dependent apoptosis cascade is initiated, causing cells to commit suicide
7. If the cell dies through necrosis, it releases toxic chemicals into the environment around it.
8. If and when the tissue is reperfused, a number of factors lead to reperfusion injury.
9. An inflammatory response is mounted, and phagocyte cells engulf damaged but still viable tissue.
Consequently results:
- reversible cell dysfunction by reperfusion which may lead finally to greater injury of the tissue ( the free radicals, especially toxic oxygen radicals are some important mediators of reperfusion injury). Further, when blood flow is restored to damaged cells, the newly-available calcium will pour through the damaged cell membranes and into mitochondria, killing cells even faster. Later, the neutrophyls, which fight disease using free radicals, accumulate at the sites of tissue injury. Of course, if blood is not restored, the tissues will die anyway.
- irreversible cell dysfunction by membrane injury, enzyme escape, calcium intracellular influx, lysososme breakdown; all of these dysfunctions lead finally to autodigestion ; after mitochondrial damage occurs the cell dysfunction is considered irreversible (if the oxidative phosphorilation is restored, the dysfunction becomes reversible) ; consequently to irreversible dysfunction eosinophylia occurs.
7. CELL AGING
A lot of cells functions decrease with aging:
- decrease of mitochondrial oxidative phosphorilation
- decrease of intracellular proteins and receptors synthesis
- decrease of reparations of chromosomal modifications.
An important aging disease is atherosclerosis which leads to decrease of blood flow and tissue hypoxia and ultimately to cell distruction.
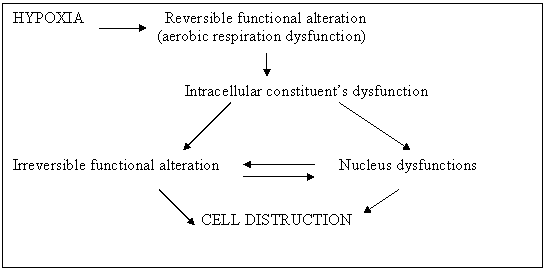
Fig. 4. Intracellular aging processes.
Aging theories
Injury factors theory (endogenous/exogenous) the aging process may occur after prolonged and repetitive exposure to exogenous factors like ionizing radiations or after decreases of defensive endogenous mechanisms like antioxidative mechanisms.
Inheritance theory the aging process is consequently to malfunction of cells, which is a genetic condition. By genetic conditions may result DNA replications errors and cells multiplication dysfunctions (this theory is not value for neurons and muscle cells which are not multiply).
8. ONCOGENESIS
Neoplasia results from disturbances of responsiveness to cells normal growth controls. The occurrences of tumoral tissues are the result of disturbances of two types of genes:
the genes who support the cellular growth pro-oncogenes genes (growth promoting genes)
the genes who controls tumor suppression anti-oncogenes genes (cancer suppression genes).
Oncogenesis theories
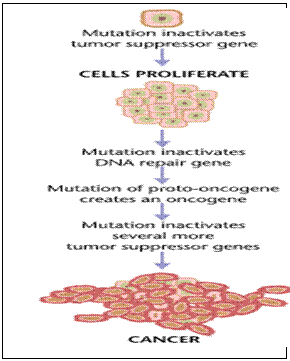 Viral theory some viruses may be
carriers of oncogenic genes ; after functional transformation these
viruses may induce in infected cells the occurrence of oncogenes signals
; a lot of viruses is considered to
be implicated in tumors occurrence: retroviruses, hepatitis B virus,
hepatitis C virus, herpes simplex type 2 virus, Epstein-Barr virus,
papillomaviruses, etc.
Viral theory some viruses may be
carriers of oncogenic genes ; after functional transformation these
viruses may induce in infected cells the occurrence of oncogenes signals
; a lot of viruses is considered to
be implicated in tumors occurrence: retroviruses, hepatitis B virus,
hepatitis C virus, herpes simplex type 2 virus, Epstein-Barr virus,
papillomaviruses, etc.The occurrence of cancer is linked by multiple gene mutations (fig).
The pathophysiological consequences of neoplasia occurrence:
the tissue histology alteration leads to functional disturbances
the tumor growth may be dangerous for the neighborhood tissues
the competition effect with the normal cells for blood supply and nutrients (the neoplasic cells have the increased metabolism)
the angiogenesis potential of tumors lead to occurrence of own vascularisation and increase the hemorrhagic risk.
9. CELL DEATH NECROSIS AND APOPTOSIS
After functional irreversible alteration of cell cellular death may result. Two forms of cellular death exist: necrosis and apoptosis.
Necrosis
Mechanism: ischemia/hypoxia, toxins, severe infections etc. Results progressive degradation of cell structure by enzymatic intracellular systems which are activated as a result of intracellular protein degradations; the particle which results from these processes are removed by phagocytes.
Apoptosis = programmed cell death
Mechanisms: physiological process (development and atrophy), certain infections, certain toxins, immune-mediated. Results activation of nuclear enzymes (by increase of intracellular calcium) and consequently lead to DNA fragmentation.
Pathological increased apoptosis can occur through the local formation of apoptotically effective mediators, the expression of their receptors, or the receptor-independent stimulation of signaling cascade. These events can be caused by ischemia (myocardial infarction, stroke), radiation or inflammation (infections, autoimmune inflammation). Apoptosis can accompanies the neuronal degeneration (Parkinsons disease, Alzheimers disease, multiple sclerosis), toxic, ischemic, and inflammatory death of liver cells (liver failure), or B cells of the pancreatic islets (type 1 diabetes mellitus), or erythropoietic cells (aplastic anemia), or lymphocytes (immunodeficiency, HIV infection).
Pathological reduced apoptosis lead to the excess of the affected cells. Among the causes are disorders of endocrine or paracrine regulation and viral infections. Absent apoptosis of virus infected cells can result in persisitent infections. Cells that escape apoptosis can develop into tumor cells. Insufficient apoptosis of immunocompetent cells is a cause of autoimmune diseases. Lack of apoptosis in pregnancy can result in abnormal embryonic or fetal development.
Pathophysiological consequences of necrosis and apoptosis: phagocytosis by neighbors, and inflammation.
10. CLINICAL ASSESMENT OF CELL INJURY AND DISEASE
clinical observation
identification of homeostasis alteration
adaptation mechanisms evaluation
etiologic and risk factors identification
pathogenetic mechanism evaluation
biological status examination (clinical assessment, paraclinical assessment blood constituents and another biological products assessment)
enzyme release assessment for cell destruction heart (myoglobin, CK, troponin, ALT, LDH), liver (AST, ALT, LDH, alkaline phosphatase), pancreas (amylase, lipase), muscle (CK, LDH, aldolasa, AST, ALT).
The relationship between cell dysfunction and disease syndrome is related in fig. 5.
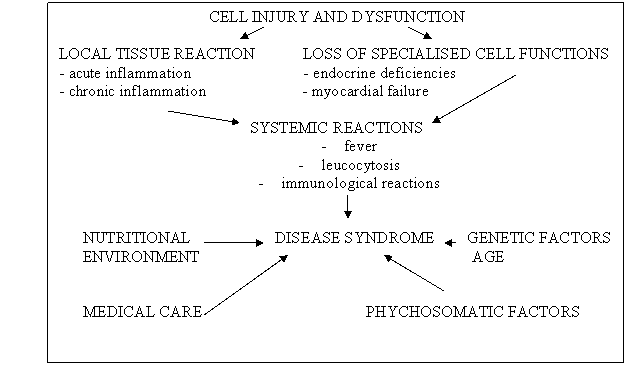
Fig. 5. The relationship between the cells dysfunctions and disease syndrome.
Healing represents the processes by which the body restores his normal functions. This process require the general response or/and local response.
Healing by general mechanism:
The response of organisms to serious injury consists in following phases:
the early phase, termed ebb phase is dominated by cardiovascular instability, alteration of circulation blood volume, impairment of oxygen transport, and heightened autonomic activity; the shock occurrence is possible in this phase
the second phase termed flow phase consist in hyperdinamic circulatory changes, fever, glucose intolerance, and muscle wasting
the final phase termed anabolic phase may persist for month and consist in mechanisms of restoration of normal functions until patient fully recovers.
Every type of mechanism of injury may lead to clinical characteristically evolution until return to healthy status.
Local healing
Local healing is the replacement of destroyed or lost tissue by viable tissue. It is achieved in two ways:
Repair the destroyed cells are replacement with connective tissue with different structural and functional characteristics (ex. wound healing)
Regeneration the destroyed cells are replacement with the same type of cells with identical structure and functions (epithelial cells regeneration, sanguine cells regeneration).
Pathological aspects of repair process:
nutritional deficiency proteins, C vitamin etc
aging
diabetes mellitus
obesity
infection
glucocorticoid therapy
inadequate blood flow (atherosclerosis, venous insufficiency)
continue immune stimulation (collagen diseases)
For the wound healing the factors who contributed the healing is ilustrated in fig. 6.
An important phenomenon of healing is neuroplasticity.
Neuroplasticity refers to the ability of the nervous system to adaptation to external and internal stimuli. After the various neurological diseases (stroke, cerebral tumors, cerebral trauma), the functional rehabilitation is based by neuroplasticity. By neuroplasticity the adjaent neurons (by morphologic and functional modifications) take the functions of injured neurons.
The efficiency of rehabilitation process depends on: patient age, the site and the size of lesion, the associative pathology, and specific rehabilitation therapy.
![]()
![]()
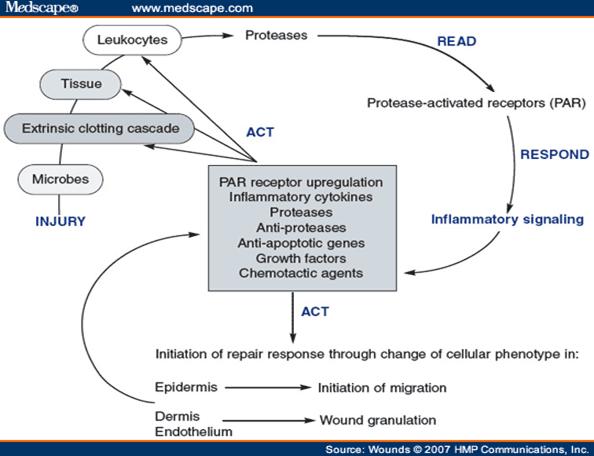
Figure 6. The cycle of injury and inflammation in the wound.
9. THE EFFECT OF INJURY AND DYSFUNCTIONS ON THE BODY
Following major tissue injury and dysfunctions systemic responses occur which are independent of the type or site of the injury or dysfunctions itself. Such responses arise, for example, following severe traumatic lesions, severe infections, extensive burns, massive hemorrhage, and major surgery. They can be considered in their temporal relationship to the time of injury.
Early reactions (first 48 h)
a. Vascular
Selective arterial constriction increase peripheral resistance and tends to compensate for diminished cardiac output. The main vessels involved are those of the skin and splanchnic circulation, whilst blood flow to the heart, brain and skeletal muscle is maintained at normal levels. When vasoconstriction fails to maintain normal blood pressure, the clinical picture of shock develops. Underperfusion of tissues leads to accumulation of acid metabolites and vessels cease to respond to normal constrictor stimuli. Progressive and irreversible arteriolar dilatation occurs and blood is sequestrated in the greatly enlarged capillary reservoir. Intractable hypotension results and this constitutes a possible lethal condition.
Main types of shock:
Hypovolemic
hemorrhage
loss of plasma (extensive burns)
loss of fluids and electrolytes (severe diarrhoea)
Cardiogenic
myocardial infarction
major pulmonary embolism
following cardiac surgery
myocarditis and other causes of acute cardiac failure
Vascular
bacteraemic
anaphylactic
neurogenic (spinal injuries)
Pathogenesis
Hypovolemia a fall in cardiac output resulting from reduced blood volume
Cardiogenic a fall in output resulting from inadequate heart function (pump failure)
Vascular mechanism
pooling of blood in a large peripheral vessels due to loss of vasomotor tone
pooling the blood in capillaries resulting from persistent venular constriction
increased vascular permeability
slowing of blood flow resulting from sludge of red blood cells
disseminated intravascular coagulation (DIC)
B. Metabolic reactions
Irreversible shock
C. Hormonal reactions
Increased production of:
catecholamines T increase cardiac output, constrict arterioles, increase gluconeogenesis
corticosteroids T retention of Na+, excretion of K+, catabolism of proteins
aldosterone T K+ deficiency
ADH T water retention
growth hormone T mobilization of adipose tissue
thyroxine T protein synthesis
Late reactions
A. Metabolic reactions
Catabolic phase
rise in oxygen consumption
rise in body temperature
catabolism of protein increased
increased mobilization of fatty acids
increased gluconeogenesis
Anabolic phase
positive nitrogen balance restored
electrolyte equilibrium regained
B. Hematologic reactions
increased formation of platelets
increased fibrinogen production
decreased plasminogen
neutrophyl leucocytosis
anemia
lymphopenia
C. Immunological reactions
reactive changes in lymphoid tissues
production of IgM antibodies directed at various components of the injured tissues.
Clinical consequences of shock
Heart - cardiac failure by subendocardial hemorrhage
Brain anoxic or hypoxic encephalopathy
Pituitary necrosis following hypovolemia giving rise to: a. acute insufficiency Sheehans syndrome b. chronic insufficiency Simmonds disease
Lungs pulmonary edema, pulmonary thrombembolism, atelectasis
Adrenals acute insufficiency ; massive hemorrhage Waterhouse-Friderichsen syndrome
Gastrointestinal tract acute ulceration of the stomach and duodenum Curlings ulcers ; hemorrhagic entheropathy resulting from hypoxia
Liver acute failure by acute necrosis
Kydney acute failure by acute tubular necrosis and microthrombosis.

|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 3477
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2026 . All rights reserved