| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
NEUROIMAGING BASIC EMOTION
How Differentiable are Basic Emotion States on the Basis of Neuroimaging Evidence?
Katherine E. Vytal
Emory University
Background
The study of human emotion is not a recent endeavor; philosophers and scientists have long sought to understand emotion and its role in thought and behavior. Notwithstanding its lengthy presence in academic and empirical ventures, emotion is far from being a well-defined psychological concept. Given the integral role of emotion in many psychological theories and clinical models, it is crucial for progress to be made toward a universally accepted definition of emotion, one that is substantiated by scientific evidence. Emotions are arguably some of the most influential forces present in human interactions; they serve to motivate behavior, modify thought, and mediate action (Barrett, 2006). They are also corollaries, responses both deliberate and involuntary to mental and physical events. Consequently, understanding emotion is central to understanding both normal and pathological functioning.
People generally claim that they can readily distinguish among certain basic emotional states such as happiness, anger and fear, suggesting that emotions are recognized and interpreted as discrete categories. A large body of evidence from behavioral, psychophysiological, and neuroscientific methods has been used to support this claim (e.g., Ekman, 1992; Damasio, Grabowski, Bechara, Damasio, Ponto, Parvizi, et al., 2000; Blair, Morris, Frith, Perrett, & Dolan, 1999). However, despite considerable support for the differentiability of emotion states, some researchers (e.g., Barrett & Russell, 1999; Lang, Bradly & Cuthbert 1990; Watson & Tellegen, 1985) have proposed that emotions are better characterized in terms of component dimensions like arousal (the extent to which an emotion evokes physiological and psychological activation) and valence (the extent to which an emotion is positive or negative), as opposed to discrete categories. By this view, discrete emotion states are not stable veridical phenomena; they are variable constructs described by common core features. Yet it also is possible, and even probable, that both component dimensions and discrete aspects can characterize emotions. Although the categorical and dimensional views of emotion have been presented as being in opposition (e.g., Barrett, 2006), they are not necessarily mutually exclusive characterizations of emotional experience. If the dimensional view of emotions is accepted, the question remains whether or not discrete emotions can be further described by stable observable patterns (e.g., neural activity or autonomic nervous system (ANS) activity). This qualifying exam seeks to establish the extent to which functional neuroimaging evidence can differentiate among discrete emotions, and evaluate how this evidence, together with psychophysiological evidence, can inform and constrain the broader theoretical debate over the existence of discrete emotion states.
Dimensional versus Discrete Debate
The dimensional approach to emotion developed out of the idea that there is a direct sequential relationship between internal sensations and the psychological interpretation of such feelings. James (1884) proposed that people do not run away from a bear because they fear it; instead they feel fear as a result of physical effects of running away. In effect, the specific ANS response (e.g., increased heart rate, increased respiration, pupil dilation, and eccrine gland secretion) to the fearful stimulus is interpreted as an emotion. This idea has been supported by studies such as (Strak, Martin, & Stepper, 1988) demonstrating that people will begin to feel an emotion (in this case, happiness) after being instructed to activate bodily states associated with that emotion (e.g., engaging facial muscles involved in smiling), an effect described by the facial feedback hypothesis.
Several researchers have used variations of the dimensional view to explain the stimulus-to-feeling sequence described by James. Schacter and Singer (1962), for example, theorized that emotions arise as a result of two factors (or dimensions): arousal and cognition. Their two-factor theory of emotion proposes that emotions are a combination of an arousal state (low to high) and the cognition that is used to best interpret such a state given a particular situation (Schacter et al., 1962). Anderson and Sobel (2003), Koronowski (1967), and Lang, Bradley, and Cuthbert (1990) all favored a biphasic theory of emotion-motivation, with arousal comprising one dimension and an appetitive-defensive scale comprising the behavior-relevant dimension. Watson and Tellegen (1985) took a similar approach to defining emotion by proposing a different two-factor theory that integrated arousal levels on both dimensions. According to their model, only the high-end of each affectively unipolar dimension (positive affect and negative affect) represents a state of emotional arousal. Comparatively, Russell (1980), Lang (1994), and Barrett and Russell (1999) proposed that emotions are defined by two dimensions, where arousal remains a separate component: arousal (low or high activation) and valence (negative to positive, or unpleasant to pleasant). By this view, an emotion like fear arises from high activation (e.g., heart rate increase) coupled with a negatively-valenced stimulus (e.g., a rabid dog).
Barrett et al. (1999) have used the dimensional model to further describe individual variation in emotional experience, suggesting that subjective experience of these dimensions is modulated by attention and incorporation of this information into the affective experience. It is in this way that Barrett et al. link their definition of emotion with their social-constructivist view on the origin of emotions; environmental input is purported to constrain the type of the emotion that is experienced based on how social demands dictate where an experience will fall on the arousal and valence dimensions. Russell (1991) reviewed an extensive amount of evidence suggesting that although emotion categories differ across cultures, they can be adequately described on three dimensions: arousal, valence, and dominance (used to capture the social power of the emotion). Russell concluded that the results of his review substantiate his claim that emotions are sociocultural linguistic constructions. Evolutionary psychologists, like Ekman (1972), have proposed that certain emotions are basic[1] (universally-experienced) products of our genetic inheritance. Rosenberg & Ekman (1995) argued against Russells claims of cross-cultural differences in emotional experience by citing methodological problems with the labeling paradigms and other methods used to evaluate the presence of emotion categories. Although the origin of emotion states is an interesting and relevant topic, it transcends the argument of whether emotions are dimensional or discrete, and it cannot be addressed by the type of analysis this examination presents. Consequently, this examination will not make any strong claims regarding the origin of emotion categories and will focus instead on the differentiability of basic emotions.
In contrast to dimensional views, discrete views have been suggested to capture more subtle differences that are essential in differentiating emotional states (Christie & Friedman, 2004). Dimensional views can be viewed as capturing higher-level aspects of emotion, particularly those that are used in evaluating subjective emotional experience. While these two characterizations are often portrayed as being in opposition, both discrete and dimensional approaches to emotion states may simply be describing different aspects of the same emotional states and are thus not necessarily mutually exclusive. Consequently, these models are considered hierarchically rather than in conflict. Ekman (1972) and others (e.g., Damasio Grabowski, Bechara, Damasio, Ponto, Parvizi et al., 2000) have claimed that certain basic emotion states are reliably separable at both the biological and the psychological level. If basic emotion states are truly differentiable, it should be possible to differentiate among them by comparing them across multiple response measures (e.g., cognitive, behavioral, physiological, neural). This exam will focus on discerning the biological patterns (physiological and neural) that may distinguish among basic emotions.
Psychophysiology
Psychophysiological and neuroscientific methods have become the two primary approaches used to evaluate the differentiability of basic emotion states at the biological level. Historically, empirical support for the discrete emotion view has been evaluated using psychophysiological measures (e.g., heart rate, galvanic skin conductance (GSC), or electroencephalogram (ECG)) that reflect ANS activity. Following the development of noninvasive neuroscientific techniques, empirical evaluation of the discrete emotion view has also been assessed at the neural level using methods such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). By examining support for the discrete emotion view from these two domains, a stronger case will be made for the idea that human affective structure includes distinct basic emotions.
In a break-through study, Ekman, Levenson, and Friesen (1983) were the first to demonstrate that emotions are differentiable on a biological level. Ekman et al. (1983) measured ANS activity (heart rate, skin temperature, GSC, and forearm flexor muscle tension) while subjects mimicked facial prototypes of emotion and recalled past emotional experiences that targeted six basic emotions (happiness, sadness, disgust, anger, fear, and surprise). Their results demonstrated that ANS activity differentiated not only between positive and negative emotions (supporting the dimensional view of emotions), but also more specifically among different negative emotions such as anger and fear (partially supporting the discrete view of emotions, and substantiating James claims of autonomic specificity). Although the results did not reveal discrete ANS signatures for all basic emotions, they did indicate that certain emotions are differentiable beyond the valence and arousal dimensions.
Recent evidence from psychophysiology has replicated and extended this valuable finding to other discrete emotion states (happiness and sadness), a result of considering multiple component factors in addition to univariate analyses (Rainville, Bechara, Naqvi, & Damasio, 2006). The conclusions of a meta-analysis Cacioppo, Bernston, Larsen, Poehlmann, and Ito (2000) prompted Rainville et al. (2006) to measure ANS responses that would better assess the relative contributions of sympathetic and parasympathetic nervous system activity in a multivariate exploratory analysis. This analysis allowed Rainville et al. to develop a heuristic decision tree from the differentiable patterns of cardiorespiratory activity observed while subjects experienced different basic emotions (happiness, sadness, anger, fear). The results clearly demonstrated that, at least on some level, physiological patterns could be used to differentiate certain emotion states.
Notwithstanding the evidence in favor of the discrete emotion view, evidence to the contrary has sparked debate over whether or not psychophysiological data can be used to differentiate among basic emotions. Meta-analyses have found that basic emotion categories cannot be consistently discriminated based solely on ANS activity (Cacioppo et al., 1997; 2000). Cacioppo et al. (1997) analyzed the results of 18 studies that used varying types of ANS measures and found some pattern reliability among emotions (e.g., anger was associated with higher diastolic blood pressure, smaller increases in cardiac output, and larger increases in peripheral resistance as compared to fear and sadness). However, the lack of overall consistency in somatovisceral patterns both with and between emotions led the authors to conclude that ANS activity alone could not differentiate among basic emotions. In their explanation, Cacioppo et al. stressed the fact that emotions may not reciprocally activate the sympathetic and parasympathetic parts of the ANS, causing the observable output measures (e.g., heart rate) to be difficult to interpret. For example, the heart rate evoked by an aversive stimulus is a product of sympathetic and parasympathetic activation, and thus it may be decelleratory or acelleratory depending on the relative activation of each branch (Berntson, Cacioppo, & Quigley, 1991). Additionally, different emotional elicitation paradigms tended to evoke different somatovisceral patterns for the same emotions (i.e., the results were not generalizable across stimulus type) (e.g., Zajonc & MacIntosh, 1992). Despite the fact that emotion states were not reliably differentiated, Cacioppo et al. claimed that the data appeared to differentiate between positive and negative evaluative systems, indicating support for the dimensional view of emotions.
The results of the Cacioppo et al. (2000) meta-analysis paralleled that of their earlier review: although some emotion-specific somatovisceral signatures were identified, they found that most somatovisceral patterns were not unique or stable. The patterns that were identified reflected highly heterogeneous data, which suggests that unspecified moderating factors might be involved, challenging the idea of a direct relationship between discrete emotion states and ANS activity (Barrett, 2006). ANS activity is known to result from the physiological demands of activity or anticipated activity, and although some emotion states have characteristic behavioral responses associated with them, emotion states and reactive behaviors do not map directly on top of one another (Barrett, 2006). Consequently, the ANS response would be highly susceptible to paradigm differences and even qualitative differences in emotion states (e.g., happiness is less likely to elicit a specific behavior than fear), suggesting that monitoring ANS activity is perhaps not the best approach to examine the structure of affect space.
Despite the transparency of the Ekman et al. versus Barrett debate, the controversy over whether or not psychophysiological evidence can and should be used to differentiate among basic emotions is not necessarily all-or-nothing. Nyklček, Thayer, and Van Doornen, (1997) was the first to approach ANS specificity with both the discrete and dimensional views using a hybrid model that incorporated the two views into one. In this hierarchical hybrid model, first described by Levenson (1998), lower-order emotion categories are characterized by higher-order dimensions of arousal and valence. By conducting a multivariate pattern classification analysis on cardiorespiratory activity, Nyklček et al. (1997) demonstrated that discrete emotions (elicited in this case by musical excerpts) represent specific locations in dimensional affective space.
Christie and Friedman (2004) also proposed a type of hybrid discrete-dimensional model to account for the inconsistencies in the psychophysiological literature and explain their own psychophysiological results (GSR, blood pressure, and ECG). Christie et al.s (2004) multivariate approach explicitly acknowledged the co-existence of discrete and dimensional structuring of affective space, in which the type of underlying dimensional structure (valence-activation or approach-withdrawal) is dependent on the manner of assessment of the emotion state (self-reported or ANS-specific). Namely, the hybrid model is proposed to function in two ways: 1) self-reported discrete emotions are situated in an affective space that is structured by a positive-negative dimensional circumplex model, and 2) emotion-specific ANS activity is situated in an affective space that is structured by an approach-withdrawal dimensional circumplex model. By structuring the affective space based on the action associated with the emotive content, this interpretation rectifies Barretts argument that ANS responses are related to behavior-oriented emotions.
Overall, psychophysiological evidence strongly suggests that at least some basic emotions are differentiable from others, particularly when multivariate analyses are used to explore the data. The prevalence of heterogeneous data, despite significant findings, suggests that ANS specificity is partially reliant on paradigm and stimulus-type as well as the extent to which the emotion in question motivates a specific behavior. However, it is impossible to ignore the results (e.g., Rainville et al.) that demonstrate the differentiability of discrete emotions despite these caveats. Future research that combines psychophysiological measure in a multivariate pattern classification analysis with other biological measures (e.g., PET and fMRI) will further clarify the differentiability of discrete emotions and the underlying structure of affective space.
Neuroimaging
Evidence from psychophysiology indicates that the relationship among emotion states may be more complex than the dimensional view by itself suggests (Rainville et al., 2006). Thus it is important to consider the extent to which basic emotions are differentiable in other related domains in order to effectively describe affective space as it relates to basic emotions. Although there appear to be differentiable patterns at the physiological level, it is not clear whether these discrete somatovisceral patterns correspond to differentiable patterns in neural systems, that is, whether there are characteristic neural systems supporting each discrete emotion. It may be the case that neural activity does not directly map onto patterns in physiology despite the fact that ANS activity is driven by the brain.
In order to determine the precise nature of the relationship between ANS activity and neural activity, future research will need to: 1) determine the neuroanatomic substrates of psychophysiological measures, 2) generate hypotheses regarding which brain regions mediate different discrete emotions based on those substrates, and 3) evaluate these hypotheses based on the current neuroimaging evidence. For example, fear is characterized by changes in heart rate coupled with changes in respiration (Cacioppo et al., 1997). The central nucleus of the amygdala has been shown to influence respiration (Takayama, Okada, & Miura, 1990), and thus may be active when an individual is in a state of fear as a result of the physiological response to an aversive stimulus. Although not within the scope of the current examination, future research that is guided by this sequence of steps will clarify the relationship between characteristic somatovisceral patterns and neural patterns and the mapping of those patterns (if they exist) onto discrete emotion states.
Although it is possible that emotion-specific brain activity does not map directly onto somatovisceral patterns, and that somatovisceral patterns do not map directly onto basic emotion states, it is unlikely that these relationships are entirely insignificant given the functional-anatomical connections of the former and the correlation between the latter. The neuroanatomic substrates of psychophysiology are well established, thus psychophysiological patterns may be a sound basis for making inferences about patterns of neural activity. The neural substrates of emotion have also been studied extensively over the past decade, though they are far from being well established.
The neural substrates of emotion were initially proposed to consist of general processing circuits, based on evidence from lesion studies in patients with midbrain and diencephalic damage (e.g., Bard, 1928; Papez, 1937). These relatively simplistic circuits were later expanded to a more complex network known as the limbic system (MacLean, 1952). Much like in the psychophysiological domain, recent evidence in neuroimaging suggests that distinct neural circuits involving both limbic and extra-limbic brain regions may support different emotion states. For example, studies in humans and animal models have strongly implicated the amygdala in the processing of fear (Davis, 1992). Using a classic fear-conditioning paradigm, where an unconditioned aversive stimulus like a shock is paired with a conditioned neutral stimulus such as a context, dozens of studies have demonstrated that the amygdala is critical for fear acquisition and expression. Lesions to the amygdala have resulted in a failure to acquire a conditioned response (fear) to the conditioned stimulus (e.g., LeDoux, Iwata, Cicchctti, & Reis, 1988) and multiple cell recording has demonstrated that amygdala cell firing is modulated when the pairing of a neutral stimulus with an aversive stimulus results in the expression of fear (Applegate, Frysinger, Kapp, & Gallagher, 1982). However, studies have shown the amygdala is not exclusively involved in fear processing, and may also support positively-valenced emotion processing (Hamann et al., 1999; Breiter et al., 1996), limiting the claims that can be made as to the unique involvement of the amygdala in the experience of fear.
Discrete emotions such as sadness and happiness have also been proposed to engage distinct neural substrates. Neuroimaging studies that used autobiographical recall mood induction have implicated subcallosal cingulate cortex (SCC) in the experiences of sadness (e.g., Mayberg et al., 1999). Additionally, resting-state studies of patients with clinical depression, a mood disorder that can cause sustained periods of sadness, have shown hypometabolism of the SCC (Mayberg, Lewis, Regenold, & Wagner, 1994). Further implicating the SCC in the experience of sadness, SCC activity has been shown to return to normal when depressed individuals successfully respond to pharmacological treatment (Mayberg et al., 2000). This series of studies indicates a critical role for the SCC in sadness as well as an integral role for the SCC in understanding clinical mood disorders like depression.
In comparison to sadness, happiness induction has been associated with activity in the basal ganglia (e.g., Whalen et al., 1998). Viewing happy facial expressions (Whalen et al., 1998), recollecting happy events (Damasio et al., 2000), and viewing pictures depicting happy scenes (Lane, Reiman, Ahern, Schwartz, & Davidson, 1997) have all been associated with increased activity in the basal ganglia. Yet just as the amygdala has exhibited a mixed association with fear and other emotion states, the basal ganglia have been implicated in the processing of withdrawal emotion states such as disgust (Philips et al., 1997), suggesting the basal ganglia is more generally involved in affective responses.
These inconsistent findings in the neuroimaging literature need to be addressed before any conclusions can be drawn as to whether or not there are patterns of neural activation that differentiate among discrete emotions. Although the idea of differentiable systems underlying different basic emotions has received support in qualitative and meta-analytic reviews, this support has been mixed, with some emotions having clearer associations with particular brain areas than others. Similar to the fact that psychophysiological literature exhibits heterogeneity and mixed results regarding basic emotions, neuroimaging results appear to be equally as challenging to interpret, although several recent reviews have made this attempt.
Existing neuroimaging meta-analyses have arrived at different conclusions regarding discrete versus dimensional views of emotion: some have found that the literature supports a dimensional view (e.g., Wager, Phan, Liberzon, & Taylor, 2003) whereas others have concluded that the literature supports a discrete view (e.g., Phan, Wager, Taylor, & Liberzon, 2002). Still others have investigated a combination of discrete and dimensional views in order to best fit the neuroimaging data (Murphy, Nimmo-Smith, & Lawrence, 2003). Importantly, it has been suggested that these inconsistencies may be a result of key methodological differences, including differences in study inclusion criteria, meta-analytic statistical methods, and criteria used for evaluating the primary theoretical questions at hand (Barrett & Wager, 2006). These and other potential problems will be evaluated in greater detail later in this examination as they become relevant.
Phan et al. (2002) conducted the first quantitative review of the neuroimaging literature examining the functional neuroanatomy of emotion. Their meta-analysis used a label-based nonparametric method, where characteristic patterns of regional activation were analyzed according to different factors of interest (similar to the basic idea behind parametric psychophysiological meta-analyses of emotion). Their primary goal was to compare patterns of regional activation across five basic emotion states (happiness, sadness, anger, fear, and disgust) as well as patterns of activation associated with negative versus positive emotions (reviewed in Wager et al., 2003). The review included 55 PET and fMRI studies that were published between January 1990 and December 2000. Emotion-specific activation coordinates were extracted from each study, standardized to a common space, and plotted on a canonical (i.e., standardized) brain that was subsequently divided into 20 orthogonal sections. For the C analyses, emotion state, valence, cognitive demand, and induction method (recall/imagery, auditory, visual) were entered separately as factors. Patterns of activity that varied reliably according to particular factors were identified and discussed.
The results of the Phan et al. meta-analysis demonstrated that certain discrete emotion states, induction methods, and task demands engaged regionally specific patterns, suggesting emotion-related functional specificity in the brain. Medial prefrontal cortex (mPFC) exhibited consistent activation across emotions indicating a general role in emotion processing regardless of emotional valence, state, or task characteristics. Emotion-specific activations revealed that fear was associated with activity in the amygdala and sadness specifically engaged the SCC. Both happiness and disgust recruited the basal ganglia, suggesting a lack of valence and emotion-related functional specificity in that region. Task-specific activations revealed that emotional induction via recall and imagery activated the anterior cingulate cortex (ACC) and insula, emotional induction by visual stimuli activated the occipital cortex and the amygdala, and tasks with cognitive demand recruited the ACC and insula. These results are consistent with animal models (e.g., Davis, 1994) and human lesion studies (e.g., Adolphs, Tranel, Damasio, & Damasio, 1994) that have previously sought to identify the role(s) of these regions in emotional experience.
In sum, Phan et al. approached the growing volume of neuroimaging data in a novel way that lent itself to addressing important questions behind the functional neuroanatomy of emotion. Although their results did not specifically evaluate the dimensional model, they were partially in favor of a discrete model, necessitating further investigation of these hypotheses. An evaluation of the dimensional model examining the effects of valence was reported separately (Wager et al., 2003) in order to devote adequate discussion to these important topics.
Wager et al. (2003) presented these additional findings in an extension of the Phan et al. meta-analysis; specifically, they investigated the extent to which dimensional views of emotion (positive-negative and approach-withdrawal) fit the neuroimaging data with respect to hemispheric lateralization of brain activity. Two opposing theories, the right-hemisphere dominance hypothesis (Bowers et al., 1985) and the valence hypothesis (Davidson, 1992) predict emotion-related hemispheric asymmetries in the brain. Evidence from patients with right hemisphere lesions has shown that the right hemisphere is critical for the ability to identify both positive and negative facial expressions (Bowers et al.). This finding is one of many that led to the development of the right-hemisphere dominance hypothesis which predicts that emotional processing is lateralized to the right hemisphere (Bowers et al., 1985). Conversely, several neuroimaging studies (e.g., Davidson, 1992; 1998) have shown that negatively-valenced emotions engage regions in the right hemisphere (e.g., right prefrontal cortex (PFC)) and positively-valenced emotions engage regions in the left hemisphere (e.g., left PFC). These findings led to the recent conceptualization of the valence hypothesis, which predicts that the right hemisphere preferentially processes emotionally negative stimuli and the left hemisphere preferentially processes emotionally posiive stimuli (Davidson, 1998). Wager et al. situated their meta-analysis of the dimensional models of emotion within the theoretical framework of the valence right hemisphere dominance hypotheses, using their findings to inform a major theoretical debate in affective neuroscience.
Additionally, Wager et al. (2003) investigated gender differences in the functional anatomy of emotion, focusing on differences in the extent of activation and the lateralization of activation in specific regions. Previous research has shown that women are more emotionally expressive (Kring & Gordon, 1988), they exhibit greater physiological responses to emotional stimuli (Orozco & Ehlers, 1998), and they exhibit different patterns of hemispheric asymmetries in emotional processing than men (Cahill et al., 2001). Consequently, their meta-analysis also explored the possibility that women and men engage distinct patterns of regional activation in response to emotional stimuli.
Wager et al. (2003) meta-analysis was structured was structured differently than Phan et al. (2002), and included 65 studies published between 1992 and February 2002. Instead of dividing the entire brain into 20 non-overlapping regions, Wager et al. defined 11 broad regions of interest (ROIs) (e.g., medial cortex: including supplementary motor cortex, mPFC, ACC, and mid- and posterior cingulate) and identified those ROIs on a canonical brain template. Peak activations were entered into the analysis in two ways: based on the author(s) anatomical labels (ROI analysis), and based on the activation coordinates reported by the author(s) (density analysis). Accordingly, reported coordinates were transformed into and plotted in Montreal Neurological Institute (MNI) space (a standardized space used for comparing activation coordinates across different subjects). Peaks were then categorized based on the three dimensions of interest: valence, approach/withdrawal, and gender.
After collapsing the ROIs into their respective hemispheres, the results of the ROI analysis did not reveal any emotion-related hemispheric effects (only the approach/withdrawal dimension was entered as a factor in this analysis). However, when considered separately, regional activations differed according to both valence and approach/withdrawal dimensions. Positive/approach emotions engaged left frontal cortex (in addition to anterior mPFC) but failed to show any association with right-lateralized activity. However, the majority of left-lateralized activations (e.g. insula, sublenticular extended amygdala, and mPFC) were associated with negative/withdrawal emotions. Withdrawal emotions also activated bilateral amygdala and striatum activation, with the majority of the striatal activation located in right caudate and putamen. Additionally, the effect of gender on emotion-related activations revealed significant findings in the whole-brain and ROI analyses. Males exhibited a greater degree of lateralization in the whole-brain density analysis, although the lateralization was region-specific. ROI analyses revealed that males showed females exhibited greater brainstem activity. Finally, basal ganglia were consistently active across valence and gender dimensions, suggesting a more general role for the basal ganglia in emotional experience.
As Wager et al. (2003) concluded, broad hemispheric divisions were too general to detect the effects of the factors considered in this meta-analysis. Overall, neural activity did not differ between hemispheres, nor did it differ consistently among regions as a function of valence or gender. Activation patterns were more complex than the hemispheric asymmetry theories predicted, suggesting that future analyses should focus on functional specificity according to discrete regional patterns rather than oversimplified neural divisions.
One notable commonality between Wager et al. and Phan et al. is the overlap between the activations associated with valence (and approach/withdrawal) and the emotion-specific activations (e.g., rostral ACC insula, and amygdala ) (Barrett et al., 2006). This relationship indicates that both the dimensional and the discrete models of emotion possess some predictive power in terms of emotion-related brain activity. Thus, it might be useful to revisit a hierarchical representation of these two models, where the dimensional model is conceptualized as a higher-order structuring of affective space. In this conceptualization, the dimensional view broadly predicts patterns of neural activation across emotions that are proximal on the dimension of interest. Conversely, the discrete view predicts more specific patterns of activation that are unique to the features of each basic emotion. Although both models hold predictive power, the discrete model might prove to be a more valuable contribution to the study of emotion because it is highly descriptive as a result of its specificity.
The third and final meta-analysis of relevance to this examination addresses the fit of both dimensional and discrete models of emotion to neuroimaging data from over 100 studies (Murphy et al., 2003). Like Wager et al. (2003), Murphy et al. evaluated the predictions of the right-hemisphere hypothesis of emotion, and investigated patterns of neural activation related to valence (positive and negative), action tendency (approach-withdrawal), and affect program emotions[2] (basic emotions: happiness, sadness, anger, fear, and disgust). A secondary analysis focused on the lateralization of neural activity as a function of valence and action tendency. Their predictions supported the co-occurrence of dimensional and discrete structuring, suggesting that both models can be used to describe characteristic aspects of emotional experience.
Similar to other neuroimaging meta-analyses (e.g., Phan et al., 2002), Murphy et al. (2003) used a label-based analysis to evaluated regional specializations for basic emotions. In addition, they extracted peak activations from the studies of interest, transformed the coordinates into MNI space, and entered them into a series of exploratory 3-D Komolgorov-Smirnov tests. Like the label-based method, the 3-D Komolgorov-Smirnov is nonparametric. However, it is more precise than the label-based method because it takes into account the 3-D pattern of activation related to a specific factor as opposed to converting the cluster of activation to a spatially arbitrary anatomic label.
In contrast to their predictions, Murphy et al. (2003) did not find any differences in activation patterns related to positive emotions or negative emotions, nor did they find any lateralization or anterior-posterior differences in neural activation related to withdrawal emotions. Approach emotions were associated with greater left-lateralized activations across anterior and posterior regions. These findings suggest the neuroimaging data do not fit well with the dimensional view. Alternatively, the discrete model of emotion was supported by the finding that characteristic neural activity in the amygdala, insula and globus pallidus, and lateral OFC was associated with fear, disgust, and anger, respectively. However, happiness and sadness did not reliably predict patterns of neural activity.
These findings parallel approach/withdrawal-related findings in Wager et al. (2003) and diverge from valence-related findings, establishing additional inconsistencies in the literature. Although both Murphy et al. (2003) and Wager et al. found that approach emotions preferentially engaged regions in the left hemisphere, Wager et al. reported activations in several regions that were not observed in Murphy et al (e.g., mPFC, insula, amygdala). Additionally, Wager et al. found that withdrawal emotions preferentially engaged regions in the right hemisphere, whereas Murphy et al. did not find any hemispheric differences in the processing of withdrawal emotions.
It is clear from this comparison that additional research and meta-analytic analyses need to be conducted in order to rectify these inconsistencies. One major obstacle in comparing across neuroimaging meta-analyses is that methodological differences make it difficult to make direct comparisons between studies. Meta-analytic methods continue to develop and improve over time, additional studies are published, and the central questions of the field and the researchers themselves tend to evolve. It is important then, to acknowledge such differences and make logical parallels where there exist so that previous findings can be recognized and extended in future research.
Current Review
In order to address the inconsistencies in the research, the current meta-analysis evaluates the extent to which neuroimaging evidence can differentiate discreet categories of emotion. Despite the fact that similar questions have been proposed in previous reviews of the basic emotion literature, the current review will differ from these reviews in four substantial respects: 1) Prior meta-analytic reviews have not considered psychophysiological and neuroimaging data together in their evaluation of evidence supporting discrete emotion theories. The current review quantitatively reviews the neuroimaging data and uses the psychophysiological data to qualitatively support this meta-analysis. 2) Neuroimaging meta-analysis methods have improved markedly in sophistication in the years following these earlier reviews. The current review uses a recently developed method, Activation Likelihood Estimation (ALE), which has considerable empirically demonstrated advantages over previously used label-based methods (Laird et al., 2005) where the anatomic locations of activations are analyzed according to their corresponding neural structure thus decreasing spatial specificity. In ALE, activation coordinates (x, y, z ) are modeled as Gaussian functions (3-D histograms that represent an estimation of the probability that each peak was active in that region) and pooled to create statistical whole-brain maps, preserving information from the original coordinates and substantially increasing the spatial quality of the analysis. 3) Existing reviews have overlooked potentially critical methodological differences, for example, the effect of including or excluding studies according to the type of baseline task (Barrett & Wager, 2006). These and other factors of interest (e.g., gender and age-range of participants, sample size, experimental paradigm (for example, fear conditioning), and stimulus presentation modality) are explicitly examined in the current review. 4) These earlier reviews do not take into account a sizeable new literature (studies published between 2003 and the present) that has resulted from the recent increase in research into the neural correlates of emotion. Approximately 124 functional neuroimaging studies were published by 1991 and currently there are over 1000 neuroimaging publications in circulation (Wager, Lindquist, & Kaplan, 2007). Thus, a substantial amount of relevant data has not been considered in previous reviews, necessitating a re-examination of the neuroimaging data in the context of a quantitative analysis. The current analysis included 35 studies published after the most recent meta-analysis (Wager et al., 2003).
In contrast to prior approaches addressing the neural substrates of emotion states (e.g., lesion studies in humans and non-human animal models), neuroimaging techniques are uniquely well suited for addressing the current state of this debate. Unlike lesion studies, which examine patients with permanent brain damage that may result in the reorganization of function, neuroimaging studies allow for the examination of neural activity across the entire brain using in vivo, non-invasive techniques.
Meta-analysis
Scope of the Review
The meta-analysis examined neuroimaging studies that included either an emotional task (e.g., mood induction) or emotional stimuli (e.g., angry facial expressions) in order to examine the neural substrates of emotional states. Like Murphy et al., (2003), this meta-analysis considered studies that addressed any aspect of an emotional experience: expression, perception, interpretation, and subjective experience. Over 1,000 potential studies were identified by a search of electronic databases (PsychInfo, MEDLINE, Web of Science ISI), Google Scholar, previous meta-analyses and relevant peer-reviewed journals. Seventy-eight neuroimaging studies (PET & fMRI) published from 1993 to 2007 were selected (See Table 1 for a summary); 35 of these studies had not been included in previous relevant meta-analyses (Phan et al., 2002; Murphy, 2003).
Studies were selected based on a set of seven criteria. First, only studies conducted using H215O PET and fMRI were considered. Second, coordinates needed to be reported in standard stereotactic space (either MNI or Talairach). Third, studies must have reported whole-brain analyses (versus ROI or VOI analyses) so that all regions of the brain had equal probability of being represented. Fourth, main effects of interest had to be reported (e.g., viewing happy faces > viewing neutral faces) so that the effects of each emotion could be analyzed independent of any other emotion. Fifth, main effects had to include at least one basic emotion state (happiness, sadness, anger, fear, or disgust). Sixth, studies had to report activations (deactivations were not included in the analysis). Seventh, all activation coordinates that were reported as significant were included in the analysis.
Data Analysis
Anatomical coordinates of emotion-specific activations were extracted from each study and entered into the meta-analysis. All MNI coordinates were converted to Talairach space using the icbm2tal transform (Lancaster et al., 2007). Individual ALE maps were calculated for each discrete emotion and pairwise comparisons were calculated by comparing two meta-analysis ALE maps (e.g., fear vs. anger) across all emotion states. This quantitative meta-analytic technique was used to localize brain regions most commonly active while subjects experienced different emotional states. Discrete emotion states (happiness, sadness, fear, anger, and disgust) were defined as differentiable if there was statistically significant agreement between an emotion state and a pattern of neural activation. Surprise was not evaluated as a basic emotion because evidence suggests that it is further divisible into positively or negatively-valenced types of surprise, making it less unitary as a discrete emotion concept (Adolphs, 2002).
The main effects of each emotion category were evaluated in the primary analysis. In the secondary analysis, five factors of interest were evaluated for each discrete emotion: Baseline (with or without a neutral baseline or otherwise comparable condition (i.e., not rest or fixation)), Gender (male, female, mixed), Age (young, defined as less than 30 years, old, defined as greater than 30 years), Paradigm (variable, but viewing facial expression, fear conditioning, and viewing emotional pictures were common examples) and Stimulus Modality (variable, but visual, auditory, imagery were common examples). Each factor was entered in a separate pair-wise contrast when there was greater than one study in a given category. The results of all main effects and contrasts (the result of subtracting one map from another) were rendered as thresholded maps (statistical whole-brain representations of the data) using GingerALE. All thresholded maps were corrected for multiple comparisons using a False Discovery Rate (FDR) algorithm, with a minimum cluster value reported at 100mm3 These maps were overlayed on a canonical brain template (the average of 27 anatomical images) and displayed using Analysis of Functional NeuroImages (AFNI) (https://afni.nimh.nih.gov/afni) (Cox, 1996).
Operational definition of differentiability
The extent to which emotions are differentiable at the neural level was evaluated using the following simple operational criterion: emotion states were considered weakly discrete if there was a significant difference between that emotion state and at least one other basic emotion state (e.g., fear vs. anger), and strongly discrete if all basic emotion states were reliably distinguished from that emotion state (e.g., all pairwise comparisons between fear and other basic emotions were significant) based on characteristic activation patterns. Differences in activation patterns among discrete emotions were evaluated by comparing observed activation distributions with null distributions (created by calculation 5,000 permutations) for each emotion. In addition to comparing between different emotion states, the most consistently active region(s) for each basic emotion will also be determined. Because the dimensional model was viewed as a higher-level approach to capturing emotion, it was not considered to be in direct opposition with the discrete model. Consequently, the meta-analysis portion of this review will not directly consider the dimensional approach, as it is not within the scope of this paper.
Results
Happiness
Main Effect[4] The main effect for happiness revealed activation clusters in the ACC, Middle Frontal Gyrus (MFG), Insula, Thalamus. and posterior cingulate (See Figure 1.)
Baseline MFG, Cingulate Gyrus
Gender Females showed an increased response to ACC, superior temporal gyrus, and inderior temploral gyrus.
Age ACC was more responsive in younger adults.
Paradigm Viewing facial expressions elicited unique activation in the fusiform gyrus.
Stimulus Modality
Sadness
Main Effect[5]
Baseline
Gender
Age
Paradigm
Stimulus Modality
Anger
Main Effect
Baseline
Gender
Age
Paradigm
Stimulus Modality
Fear
Main Effect Main effect of fear elicited amygdala activation
Baseline
Gender
Age
Paradigm
Stimulus Modality
Disgust
Main Effect The main effect for disgust revealed activation clusters in the inferior frontal gyrus, medial gyrus, ACC, middle temporal gyrus, (See Figure 5.)
Baseline
Gender
Age
Paradigm
Stimulus Modality
Conclusions
Meta-analysis
The primary goal of this review was to assess the extent to which neuroimaging evidence is differentiable based on evidence from PET and fMRI. Results from the meta-analysis revealed several important findings. First,
Certain emotion states were shown to be more differentiable (e.g., Anger and
A secondary goal of the analysis was to qualitatively review the psychophysiological literature and compare the findings with the results from the meta-analysis. By establishing that certain discrete emotion states are more or less differentiable than others on the basis of the psychophysiological neuroimaging evidence, valuable parallels can be made between the two domains. These parallels may serve to constrain our understanding of emotion by informing theoretical accounts of how affective space is structured. Reviews of the psychophysiological literature (e.g., Cacioppo et al., 1997; Rainville et al., 2006) have demonstrated that certain basic emotions (e.g., fear and anger) exhibit characteristic somatosensory signatures, while others (e.g., happiness) exhibit more variable physiological responses. Similarly, meta-analyses of emotion (e.g., Phan et al., 2002) have demonstrated that some basic emotions (e.g., fear and sadness) engage distinct neural substrates (Amygdala and SCC, respectively), suggesting that certain emotion states are discrete on a physiological, neurological, and psychological level.
Caveats and Limitations
Given that the meta-analysis of neuroimaging data and neuroimaging itself is in its infancy, a few limitations are inherent in this type of review. Spatial localization is a primary concern. Neuroimaging data have moderate spatial resolution (approximately 3 cubic mm), but preprocessing steps often include smoothing the data, which further distorts the location and extent of activations. Additional spatial information is lost in the transition from data acquisition to publication because most studies report only the peak activation coordinates from an active cluster. This practice ignores information regarding the spatial extent of activations and the possibility that there are other activations in that particular cluster that approach the strength of that of the peak. Consequently, the data that are available to the scientific community may lack spatial information that is highly relevant from the standpoint of a quantitative review.
In addition, neuroimaging analyses exhibit considerable variability in the level of statistical thresholds they set and how (if at all) they correct for multiple comparisons in their statistical maps. The number of cubic volumes (voxels) analyzed in a typical brain is approximately 50,000, and if the threshold is set at .01, around 500 of those voxels will appear to exhibit activation simply by chance. The problem of multiple comparisons increases as the threshold becomes less stringent; for instance, if a threshold of .05 is used, approximately 2,500 voxels will exhibit spurious activation. There are several approaches to correcting for multiple comparisons, and they range in increasing stringency from the false discovery rate (FDR) correction (where spurious effects may be present) to the Bonferroni correction (where true effects may be lost). Depending on the statistical threshold set and the correction method used, the same dataset can produce drastically different results. Without a standard statistical threshold, it is impossible to assess the true distribution of whole brain activations across studies. In the future, this problem should be addressed at the individual study level (by adhering to a universal standard) and eventually at the level of neuroimaging meta-analytic techniques (i.e., studies that violate this standard should be excluded or given less weight in the analysis).
There are also several limitations regarding the scope and generalizability of the meta-analysis itself. A large number of studies were excluded from this review because of missing information, reducing power in the analysis and the generalizability. For example, some studies neglected to report whole-brain analyses, activation coordinates, and/or main effects of interest (e.g., Happy > Neutral) that were necessary components of this particular analysis. Within those studies that were included, differences in task-type and stimulus-type made direct comparison difficult. However, the results of paradigm and task modality factor analyses indicate that there are no systematic patterns in the distribution of activations according to these factors. In addition, the analysis likely characterized a more ecologically valid response as a result of the task and stimulus diversity (e.g., conditioned fear-learning versus music-induced mood) present in the studies analyzed. Homogenous data lend themselves to detecting statistical effects, but the results of a meta-analysis are less generalizable if they are the product of data that only examines the effects of a specific paradigm or stimulus. Outside of the lab, emotional responses are not locked to one type of stimulus (e.g., fearful responses are not restricted situations where one observes another person with a fearful facial expression), so it makes sense to study emotions across a wide range of contexts.
Despite these limitations, it is still very important to pursue the analysis and observation of global data trends in order to address the theoretical questions that remain regarding emotion. The benefits of meta-analysis and advantages of neuroimaging techniques far out-weigh the limitations associated with them. Neuroimaging affords researchers a noninvasive window into the brain, allowing questions about functional specialization and organization to be addressed in ways that were not available two decades ago. Recent advances in neuroimaging meta-analytic methods (e.g., ALE) have provided researchers with the tools to analyze trends across a large number of studies, while preserving spatial information and allowing for the creation of parametric statistical maps (versus label-based distributions). As the technology behind neuroimaging increases in sophistication (e.g., spatial resolution improves), and additional methods of neuroimaging meta-analysis develop (e.g., multivariate statistical tests), the theoretical impact of this type of review will have an even more profound effect on how we conceptualize emotion.
Implications and Future Directions
General Discussion
Neuroimaging analysis is not phrenology: Specific localized regions are working in concert as part of functionally separable systems. Psychophysiological data have been used to differentiate emotions in the past, and with the development of new methods to analyze existing neuroimaging data we can answer questions that could not be previously addressed.
This review quantitatively evaluated the evidence supporting the idea that basic emotion states are associated with distinct neural signatures, and qualitatively examined whether or not these patterns matched the patterns in the psychophysiological data. The results of the meta-analysis indicate that disgust and fear are moderately differentiable based on the evidence When these patterns are mapped onto the patterns in the psychophysiological data, it is evident that there is at least some common structure between the two domains, although the relationship is certainly not a one-to-one mapping.
Much like the current review, previous meta-analyses (e.g., Phan et al., 2002; and Murphy et al., 2003) have reported mixed results in support of a discrete emotion view. It appears from the comparison of these three analyses that neuroimaging evidence does not cleanly and equally differentiate among basic emotions. However, that is not to say the discrete emotion view should be completely disregarded; the idea that some basic emotions elicit characteristic physiological and neural responses is supported by the literature. The lack of clarity may also result from the fact that these reviews all targeted highly similar datasets, which may have led to similar problems that were inherent in the dataset (and may be rectified with the exclusion of studies that exhibit marked inconsistencies).
Alternatively, even if these effects are not confounded by spurious data, the previous reviews have demonstrated that it is possible to differentiate among basic emotions based on physiological (e.g., Rainville et al., 2006) and neurological (e.g., Phan et al.) evidence, suggesting that there are some discrete emotion states that exhibit characteristic response patterns. Given that strict dimensional theorists (e.g., Barrett et al., 2006) deny that emotional states are differentiable on any level, this evidence indicates that a strict dimensional view is not adequate for characterizing affective space as it relates to emotional categories. A two-dimensional structure of affect does not fit these results well because it cannot account for the qualitative differences that differentiate discrete basic emotions. For instance, a particular state of fear is not necessarily more or less arousing than one of anger, nor is it necessarily more or less negative. It is not only quantitative differences (i.e., differences in arousal or valence) but also the qualitative differences that distinguish basic emotions. To reiterate a previous claim, dimensional characterizations may be better at describing higher-level emotional categories, whereas discrete views may be better at describing lower-level categorical boundaries.
Support for the organization of emotions in a dimensional affective space has also been variable as previous noted, although this variability may be specific to the modality in which the patterns are being observed. Results have been mixed where neuroimaging meta-analyses have evaluated support for the dimensional view (e.g., Wager et al., 2003; Murphy et al., 2003) suggesting that perhaps a hybrid or alternate organization of affective space might more closely predict the patterns in the data. Conversely, psychophysiological meta-analyses report consistent effects in favor of patterns differentiating dimensions such as valence and approach-withdrawal (Cacioppo et al., 2000). These findings may indicate that the psychophysiological data are capturing global effects and the neuroimaging data are capturing more variable effects of emotion states. However, it must also be noted that the dimensional factors have not in fact been analyzed as dimensions in these analyses, instead they have been analyzed a categorical factors (e.g., positive and negative versus on a scale). The decision to analyze dimensional effects on the two extremes of the dimension could bias these analyses either in favor of the dimensional view when a more categorical organization exists, or against the dimensional view because the range of values on which different emotion states fall is restricted to the boundaries of the scale. Additionally, traditional dimensional views involve et least two dimensions arranged in a circumplex model. As of yet, reviews have targeted one or two overlapping dimensions (e.g., valence and approach-withdrawal) as opposed to complementary dimensions that comprise such a model (e.g.., valence and arousal). Future review should attempt to examine complementary dimensions in order to evaluate the dimensional models more directly.
It is also important to consider that despite general trends in thought and behavior, many psychological responses (and likewise, physiological and neurological responses) are situation-dependent. Barsalou (2003) has shown that mental simulations in the conceptual system vary considerably based on context, and it follows that action-oriented emotional categories (e.g., anger) would have characteristic responses that are specific to a given situation. According to Barsalou, psychological concepts are dynamical because they are action-oriented, meaning they have the capacity to simulate a variety of different instances in which a person may be required to act. Similarly, a given emotion state may also be dynamical and the resulting response (physiological, neurological, psychological) may be adapted according to the situation at hand. If this is the case, it may be more important then to consider for each emotion what conditions elicit differentiable behavior, physiological responses, and neural activity. Conceptualizing the relationship among these variables and different emotion states as dynamic rather than invariant might prove to be more productive (Cacioppo et al., 1997).
Defining the organization of affective space is particularly important on both a psychological and a neurobiological level because of the implications it has on clinical theory and the understanding of mood disorders. Individuals with major depressive disorder (MDD) exhibit increased activation in areas of the brain (e.g., insula, amygdala , ACC) that are associated with the processing of negative emotions like fear and sadness (Beauregard, Paquette, and Levesque, 2006). Clinical Implications..DSM-IV criteria for depression,.
Future Directions
Accordingly, future research should consider a dynamic, multidimensional, multivariate approach to characterizing basic differentiable emotion states. Success using multivariate analysis with psychophysiology (e.g., Rainville et al., 2006) suggests that multivariate analysis of fMRI data might reveal more complex patterns differentiating emotions, better targeting the complex nature of responses associated with different emotion states. However, this type of analysis has been neither extensively developed nor validated in the literature and may be a promising future step in the pursuit of defining emotions and describing affective space. It is also particularly importance to address the problem of statistical threshold variation across neuroimaging studies, because this factor can have a substantial impact on the number of active coordinates a study reports. Neuroimaging analysis methods should be standardized so that a single studys results can be readily compared to similar work in the greater scientific community. Additionally, meta-analytic methods should explicitly consider the sample size, the spatial extent of activations, and the neuroimaging design methodology (e.g., event-related, block) of studies because these factors can directly impact statistical power, spatial localization (e.g., Wager, Lindquist, & Kaplan, 2007), and the probability that the activations reported are meaningful.
Does psychological differentiability engender neural differentiability (e.g., fear is more psychologically distinct from other discrete emotions; is fear equally as distinct at the neural level?)
Only after emotion states are better characterized in healthy individuals will progress be made towards understanding emotional disorders (e.g., MDD) in clinical populations, eventually leading to earlier interventions and more effective treatment methods for such individuals. Despite prior emphasis on emotion as a unified concept, emotion appears to be much more dynamic, with a complex organizational structure. Ideally a multidimensional model of affective space will be developed, one that accurately describes the variable and characteristic features of emotion states.
Psychology reflected in physiology (ANS & neural)
Future consider .
Self and others
The ability to produce and distinguish different basic emotional states (e.g., happiness, anger, and fear) is adaptive
References
Aalto, S., Naatanen, P., Wallius, E., Metsahonkala, L., Stenman, H., Niemi, P. M., et al. (2002). Neuroanatomical substrata of amusement and sadness: a PET activation study using film stimuli. Neuroreport, 13(1), 67-73.
Abel, K. M. C. A., Allin, M. P. G., Kucharska-Pietura, K., David, A., Andrew, C., Williams, S., et al. (2003). Ketamine alters neural processing of facial emotion recognition in healthy men: an fMRI study. Neuroreport, 14(3), 387-391.
Abler, B., Erk, S., Herwig, U., & Walter, H. (2007). Anticipation of aversive stimuli activates extended amygdala in unipolar depression. Journal of Psychiatric Research, 41(6), 511-522.
Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372,669-672.
Applegate, C. D., Frysinger, R. C., Kapp, B. S., & Gallagher, M. (1982). Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Research, 238, 457-462.
Armony, J. L. C. A., & Dolan, R. J. (2001). Modulation of auditory neural responses by a visual context in human fear conditioning. Neuroreport, 12(15), 3407-3411.
Baker, S. C., Frith, C. D., & Dolan, R. J. (1997). The interaction between mood and cognitive function studied with PET. Psychological Medicine, 27, 56578.
Benuzzi, F., Meletti, S., Zamboni, G., Calandra-Buonaura, G., Serafini, M., Lui, F., et al. (2004). Impaired fear processing in right mesial temporal sclerosis: a fMRI study. Brain Research Bulletin, 63(4), 269-281.
Barrett, L. F. (2006). Are emotions natural kinds?. Psychological Science, 1(1), 28-58.
Barsalou, L.W. (2003). Situated simulation in the human conceptual system. Language and Cognitive Processes, 18, 513-562.
Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98, 459-487.
Blair, R. J. R., Morris, J. S., Frith, C. D., Perrett, D. I., & Dolan, R. J. (1999). Dissociable neural responses to facial expressions of sadness and anger. Brain, 122, 883-893.
Breiter, H. C., Etcoff, N. L., Whalen, P. J., Kennedy, W. A., Rauch, S. L., Buckner, R. L., et al. (1996). Response and Habituation of the Human Amygdala during Visual Processing of Facial Expression. Neuron, 17(5), 875-887.
Bowers D., Bauer R. M., Coslett H. B., & Heilman K. M. (1985). Processing of face by patients with unilateral hemisphere lesions: dissociations between judgments of facial affect and facial identity. Brain and Cognition, 4, 258-272.
Buchanan, T. W., Lutz, K., Mirzazade, S., Specht, K., Shah, N. J., Zilles, K., et al. (2000). Recognition of emotional prosody and verbal components of spoken language: an fMRI study. Cognitive Brain Research, 9(3), 227-238.
Buchel, C., Dolan, R. J., Armony, J. L., & Friston, K. J. (1999). Amygdala-Hippocampal Involvement in Human Aversive Trace Conditioning Revealed through Event-Related Functional Magnetic Resonance Imaging. Journal of Neuroscience, 19(24), 10869-10876.
Buchel, C., Morris, J., Dolan, R. J., & Friston, K. J. (1998). Brain Systems Mediating Aversive Conditioning: an Event-Related fMRI Study. Neuron, 20(5), 947-957.
Cacioppo, J. T., Berntson, G. G., Klein, D. J., & Poehlmann, K. M. (1997). The psychophysiology of emotion across the lifespan. Annual Review of Gerontology and Geriatrics, 17, 27-74.
Cahill, L., Haier, R. J., White, N. S., Fallon, J., Kilpatrick, L., Lawrence, C., Potkin, S. G., & Alkire, M. T. (2001). Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory, 75(1), 19.
Christie, I. C., & Friedman, B. H. (2004). Autonomic specificity of discrete emotion and dimensions of affective space: A multivariate approach. International Journal of Psychophysiology, 51, 143-153.
Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162-173.
Damasio, A. R., Grabowski, T. J., Bechara, A., Damasio, H., Ponto, L. L. B., Parvizi, J., and Hichwa, R. D. (2000). Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience, 3, 10491056.
Davidson, R.J., (1998). Affective style and affective disorders: perspectives from affective neuroscience. Cognition and Emotion, 12, 307330.
Davidson, R.J., (1992). Anterior cerebral asymmetry and the nature of emotion. Brain Cognition, 20(1), 125151.
Davidson, R. J., Abercrombie, H., Nitschke, J. B., & Putnam, K. (1999). Regional brain function, emotion, and disorders of emotion. Current Opinion in Neurobiology, 9, 228-234.
Dolan, R. J., Fletcher, P., Morris, J., Kapur, N., Deakin, J. F. W., & Frith, C. D. (1996). Neural Activation during Covert Processing of Positive Emotional Facial Expressions. NeuroImage, 4, 194-200.
Dougherty, D. D., Shin, L. M., Alpert, N. M., Pitman, R. K., Orr, S. P., Lasko, M., et al. (1999). Anger in healthy men: a PET study using script-driven imagery. Biological Psychiatry, 46(4), 466-472.
Fischer, H., Sandblom, J., Gavazzeni, J., Fransson, P., Wright, C. I., & Backman, L. (2005). Age-differential patterns of brain activation during perception of angry faces. Neuroscience Letters, 386(2), 99-104.
Fitzgerald, D. A., Posse, S., Moore, G. J., Tancer, M. E., Nathan, P. J., & Phan, K. L. (2004). Neural correlates of internally-generated disgust via autobiographical recall: a functional magnetic resonance imaging investigation. Neuroscience Letters, 370(2-3), 91-96.
Fredrikson, M., Furmark, T., Olsson, M. T., Fischer, H., Andersson, J., & Langstrom, B. (1998). Functional neuroanatomical correlates of electrodermal activity: A positron emission tomographic study. Psychophysiology, 35(2), 179-185.
George, M. S., Ketter, T. A., Parekh, P. I., Herscovitch, P., & Post, R. M. (1996). Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biological Psychiatry, 40(9), 859-871.
George, M. S., Ketter, T. A., Parekh, P. I., Horwitz, B., Herscovitch, P., & Post, R. M. (1995). Brain Activity During Transient Sadness and Happiness in Healthy Women. American Journal of Psychiatry, 152(3), 341-351.
Grosbras, M. H. & Paus, T. (2005). Brain networks involved in viewing angry hands or faces. Cerebral Cortex, 12.
Habel, U., Klein, M., Kellermann, T., Shah, N. J., & Schneider, F. (2005). Same or different? Neural correlates of happy and sad mood in healthy males. NeuroImage, 26(1), 206-214.
Hariri, A. R., Mattay, V. S., Tessitore, A., Fera, F., & Weinberger, D. R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry
Harris, L. T., & Fiske, S. T. (2007). Dehumanizing the lowest of the low: Neuroimaging responses to extreme outgroups. Psychological Science, 17(10), 847-853.
James, W. (1884). What is an emotion? Mind, 19, 188 205.
Kesler/West, M. L., Andersen, A. H., Smith, C. D., Avison, M. J., Davis, C. E., Kryscio, R. J., et al. (2001). Neural substrates of facial emotion processing using fMRI. Cognitive Brain Research, 11(2), 213-226.
Kimbrell, T. A., George, M. S., Parekh, P. I., Ketter, T. A., Podell, D. M., Danielson, A. L., et al. (1999). Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biological Psychiatry, 46(4), 454-465.
Kring, A. M., & Gordon, A. H., (1998). Sex differences in emotion: expression, experience, and physiology. Journal of Personality and Social Psychology. 74(3), 686 703.
LaBar, K. S., Crupain, M. J., Voyvodic, J. T., & McCarthy, G. (2003). Dynamic Perception of Facial Affect and Identity in the Human Brain. Cerebral Cortex, 13(10), 1023-1033.
Lancaster, J. L., Tordesillas-Gutierrez, D., Martinez, M., Salinas, F., Evans, A., Zilles, K., Mazziotta, J. C., Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping.
Laird, A. M., Fox, P. M., Price, C. J., Glahn, D. C., Uecker, A. M., Lancaster, J. L.,. Turkeltaub, P. E., Kochunov, P., & Fox, P.T. (2005). ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping, 25(1), 155-164.
Lane, R. D., Reiman, E. M., Ahern, G. L., Schwartz, G. E., & Davidson, R. J. (1997). Neuroanatomical correlates of happiness, sadness, and disgust. American Journal of Psychiatry, 154, 926933.
Lang, P. J.(1994). The varieties of emotional experience: A meditation of James-Lange theory. Psychological Review, 101, 211-221.
Lange, K., Williams, L. M., Young, A. W., Bullmore, E. T., Brammer, M. J., Williams, S. C. R., Gray, J. A., & Phillips, M. L. (2003) Task instructions modulate neural responses to fearful facial expressions. Biological Psychiatry, 226232
LeDoux, J. E., Iwata, J., Cicchctti, P., & Reis, D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience, 8, 2517-2529.
Lemche, E. a. e., Surguladze, S. A. a., Giampietro, V. P. b., Anilkumar, A. a., Brammer, M. J. b., Sierra, M. c., et al. (2007). Limbic and prefrontal responses to facial emotion expressions in depersonalization. Neuroreport, 18(5), 473-477.
Lennox, B. R., Jacob, R., Calder, A. J., Lupson, V., & Bullmore, E. T. (2004). Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Medicine, 34(5), 795-802.
Liotti, M., Mayberg, H. S., Brannan, S. K., McGinnis, S., Jerabek, P., & Fox, P. T. (2000). Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biological Psychiatry, 48(1), 30-42.
Mayberg, H. S., Lewis, P. J., Regenold, W., & Wagner, H. N. Jr. (1994). Paralimbic hypoperfusion in unipolar depression. Journal of Nuclear Medicine, 35, 929934.
Mayberg, H. S., Liotti, M., Brannan, S. K., McGinnis, S., Mahurin, R. K., Jerabek, P. A., Silva, J. A., Tekell, J. L., Martin, C. C., Lancaster, J. L., & Fox, P. T. (1999). Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry, 156, 67582.
Mayberg, H. S., Brannan, S. K., Tekell, J. L., Silva, J. A., Mahurin, R. K., McGinnis, S., & Jerabek, P. A. (2000). Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biological Psychiatry, 48, 830843.
Mitterschiffthaler, M. T., Fu, C. H. Y., Dalton, J. A., Andrew, C. M., & Williams, S. C. R. (in press). A functional MRI study of happy and sad affective states induced by classical music. Human Brain Mapping, 177-182.
Moll, J., de Oliveira-Souza, R., & Eslinger, P. J. (2003). Morals and the human brain: A working model. Neuroreport, 14(3), 299-305.
Morris, J. S., Friston, K. J., & Dolan, R. J. (1997). Neural Responses to Salient Visual Stimuli. Proceedings: Biological Sciences, 264(1382), 769-775.
Morris, J. S., Friston, K. J., Buchel, C., Frith, C. D., Young, A. W., Calder, A. J., et al. (1998a). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121(1), 47-57.
Morris, J. S. (1998b). Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proceedings of the Royal Society of London. Series B, Biological Sciences, 265(1397), 649-657.
Morris, J. S., Ohman, A., & Dolan, R. J. (1999). A subcortical pathway to the right amygdala mediating 'unseen' fear. PNAS, 96(4), 1680-1685.
Morris, J. S., Buchel, C., & Dolan, R. J. (2001). Parallel Neural Responses in Amygdala Subregions and Sensory Cortex during Implicit Fear Conditioning: Volume 13, Number 6 (2001), pages 1044-1052. NeuroImage, 14(2), 529.
Nitschke, J. B., Sarinopoulos, I., Mackiewicz, K. L., Schaefer, H. S., & Davidson, R. J. (2006). Functional neuroanatomy of aversion and its anticipation. NeuroImage, 29(1), 106-116.
Orozco, S., & Ehlers, C. L.(1998). Gender differences in electrophysiological responses to facial stimuli. Biological Psychiatry, 44(4), 281289.
Ottowitz, W. E., Dougherty, D. D., Sirota, A., Niaura, R., Rauch, S. L., & Brown, W. A. (2004). Neural and Endocrine Correlates of Sadness In Women: Implications for Neural Network Regulation of HPA Activity. Journal of Neuropsychiatry: Clinical Neuroscience, 16(4), 446-455.
Paradiso, S., Robinson, R. G., Andreasen, N. C., Downhill, J. E., Davidson, R. J., Kirchner, P. T., et al. (1997). Emotional activation of limbic circuitry in elderly normal subjects in a PET study. American Journal of Psychiatry, 154(3), 384-389.
Paradiso, S., Robinson, R. G., Boles Ponto, L. L., Watkins, G. L., & Hichwa, R. D. (2003). Regional Cerebral Blood Flow Changes During Visually Induced Subjective Sadness in Healthy Elderly Persons. Journal of Neuropsychiatry: Clinical Neuroscience, 15(1), 35-44.
Pardo, J. V., Pardo, P. J., & Raichle, M. E. (1993). Neural correlates of self-induced dysphoria. The American Journal of Psychiatry, 150(5), 713.
Pelletier, M., Bouthillier, A., Levesque, J., Carrier, S., Breault, C., Paquette, V., et al. (2003). Separate neural circuits for primary emotions? Brain activity during self-induced sadness and happiness in professional actors. Neuroreport, 14(8), 1111-1116.
Phelps, E. A., O'Connor, K. J., Gatenby, J. C., Gore, J. C., Grillon, C., & Davis, M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4(4), 437-441.
Phillips, M. L., Bullmore, E. T., Howard, R., Woodruff, P. W. R., Wright, I. C., Williams, S. C. R., et al. (1998). Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Research: Neuroimaging, 83(3), 127-138.
Phillips, M. L., Drevets, W. C., Rauch, S. L., Lane, R.D. (2003). Neurobiology of emotion perception. I: The neural basis of normal emotion perception. Biological Psychiatry, 54, 50414
Phillips, M. L., Marks, I. M., Senior, C., Lythgoe, D., ODwyer, A. M., Meehan, O., Williams, S. C. R., Brammer, M. J., Bullmore, E. T., & Mc Guire, P. K. (2000). A differential neural response in obsessive-compulsive disorder patients with washing compared with checking symptoms to disgust. Psychological Medicine, 30, 1037-1050.
Phillips, M. L., Williams, L. M., Heining, M., Herba, C. M., Russell, T., Andrew, C., et al. (2004). Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. NeuroImage, 21(4), 1484-1496.
Phillips, M. L., Young, A. W., Scott, S. K., Calder, A., Andrew, C., Brammer, M., Giampietro, V., Williams, S. C. R., Bullmore, E. T., Brammer, M., & Gray, J. A. (1999). Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Royal Society of London: Series B, 265, 1809-1817.
Phillips, M. L., Young, A. W., Senior, C., Brammer, M., Andrew, C., Calder, A. J., Bullmore, E. T., Perrett, D. I., Rowland, D., Williams, S. C., Gray, J. A., & David, A. S. (1997). A specific neural substrate for perceiving facial expressions of disgust. Nature, 389, 495-498.
Pietrini, P., Guazzelli, M., Basso, G., Jaffe, K., & Grafman, J. (2000). Neural Correlates of Imaginal Aggressive Behavior Assessed by Positron Emission Tomography in Healthy Subjects. American Journal of Psychiatry, 157(11), 1772-1781.
Pine, D. S., Grun, J., Zarahn, E., Fyer, A., Koda, V., Li, W., et al. (2001). Cortical brain regions engaged by masked emotional faces in adolescents and adults: an fMRI study. Emotion, 1(2), 137-147.
Rosenberg, E., & Ekman, P. (1995). Conceptual and methodological issues in the judgment of facial expression of emotion. Motivation and Emotion, 19, 111-138.
Sambataro, F., Dimalta, S., Di Giorgio, A. et al. (2006) Preferential responses in amygdala and insula during presentation of facial contempt and disgust. European Journal of Neuroscience, 24, 23552362.
Sato, W., Kochiyama, T., Yoshikawa, S., Naito, E., & Matsamura, M. (2004). Enhanced neural activity in response to dynamic facial expression of emotion: an fMRI study. Cognition and Brain Research, 20, 8191.
Schafer, A., Schienle, A., & Vaitl, D. (2005). Stimulus type and design influence hemodynamic responses towards visual disgust and fear elicitors. International Journal of Psychophysiology, 57(1), 53-59.
Schienle, A. C., Schafer, A., Stark, R., Walter, B., & Vaitl, D. (2005). Gender differences in the processing of disgust- and fear-inducing pictures: an fMRI study. Neuroreport, 16(3), 277-280.
Schienle, A. C., Stark, R., Walter, B., Blecker, C., Ott, U., Kirsch, P., et al. (2002). The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport, 13(16), 2023-2026.
Shapira, N. A., Liu, Y., He, A. G., Bradley, M. M., Lessig, M. C., James, G. A., et al. (2003). Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biological Psychiatry, 54(7), 751-756.
Sprengelmeyer, R., Schroeder, U., Young, A. W., & Epplen, J. T. (2006). Disgust in pre-clinical Huntington's disease: a longitudinal study. Neuropsychologia, 44, 51833.
Stark, R., Schienle, A., Sarlo, M., Palomba, D., Walter, B., & Vaitl, D. (2005). Influences of disgust sensitivity on hemodynamic responses towards a disgust-inducing film clip. International Journal of Psychophysiology, 57(1), 61-67.
Stark, R., Schienle, A., Walter, B., Kirsch, P., Sammer, G., Ott, U., et al. (2003). Hemodynamic responses to fear and disgust-inducing pictures: an fMRI study. International Journal of Psychophysiology, 50(3), 225-234.
Strak, F., Martin, L. L., & Stepper, S. (1988). Inhibiting and facilitating conditions of facial expressions: a non-obtrusive test of the facial feedback hypothesis. Journal of Personality and Social Psychology, 54, 768-777.
Tabbert, K., Stark, R., Kirsch, P., & Vaitl, D. (2005). Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. International Journal of Psychophysiology, 57(1), 15-23.
Thielscher, A., & Pessoa, L. (2007). Neural Correlates of Perceptual Choice and Decision Making during Fear-Disgust Discrimination. Journal of Neuroscience, 27(11), 2908-2917.
Vuilleumier, P., & Pourtois, G. (2007). Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia, 45(1), 174-194.
Wager, T. D., Lindquist, M., & Kaplan, L. (2007). Meta-analysis of functional neuroimaging data: Current and future directions. Social, Cognitive, and Affective Neuroscience, 2(2), 150-158.
Wang, L., McCarthy, G., Song, A. W., & LaBar, K. S. (2005). Amygdala Activation to Sad Pictures During High-Field (4 Tesla) Functional Magnetic Resonance Imaging. Emotion, 5(1), 12-22.
Whalen, P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B., & Jenike, M. A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411418.
Wicker, B., Keysers, C., Plailly, J., Royet, J.-P., Gallese, V., & Rizzolatti, G. (2003). Both of Us Disgusted in My Insula: The Common Neural Basis of Seeing and Feeling Disgust. Neuron, 40(3), 655-664.
Williams, L. M. C. A., Das, P., Liddell, B., Olivieri, G., Peduto, A., Brammer, M. J., et al. (2005). BOLD, sweat and fears: fMRI and skin conductance distinguish facial fear signals. Neuroreport, 16(1), 49-52.
Williams, L. M., Phillips, M. L., Brammer, M. J., Skerrett, D., Lagopoulos, J., Rennie, C., et al. (2001). Arousal Dissociates Amygdala and Hippocampal Fear Responses: Evidence from Simultaneous fMRI and Skin Conductance Recording. NeuroImage, 14(5), 1070-1079.
Winston, J. S., Vuilleumier, P., & Dolan, R. J. (2003). Effects of Low-Spatial Frequency Components of Fearful Faces on Fusiform Cortex Activity. Current Biology, 13(20), 1824-1829.
Wright, P., He, G., Shapira, N. A., Goodman, W. K., & Liu, Y. C. (2004). Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport, 15(15), 2347-2351.
Zajonc, R. B., & McIntosh, D. N. (1992). Emotions research: Some promising questions, some questionable promises. Psychological Science, 3, 70-74.
Appendix A
Acronym Glossary
ACC Anterior Cingulate Cortex
AFNI Analysis of Functional NeuroImages
ALE Activation likelihood estimation
ANS Autonomic nervous system
fMRI Functional magnetic resonance Imaging
GP Globus Pallidus
MDD Major depressive disorder
MFG Middle Frontal Gyrus
mPFC Medial prefrontal cortex
MNI Montreal Neurological Institute
OFC Orbitofrontal Cortex
PET Positron emission tomography
PFC Prefrontal cortex
ROI Region of Interest
SCC Subcallosal cingulate cortex
VOI Volume of Interest
Table 1. Summary of studies included in this review
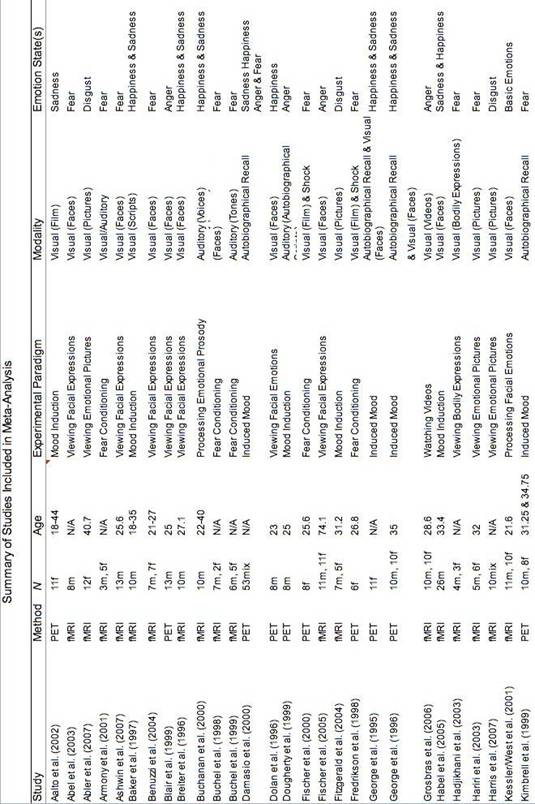

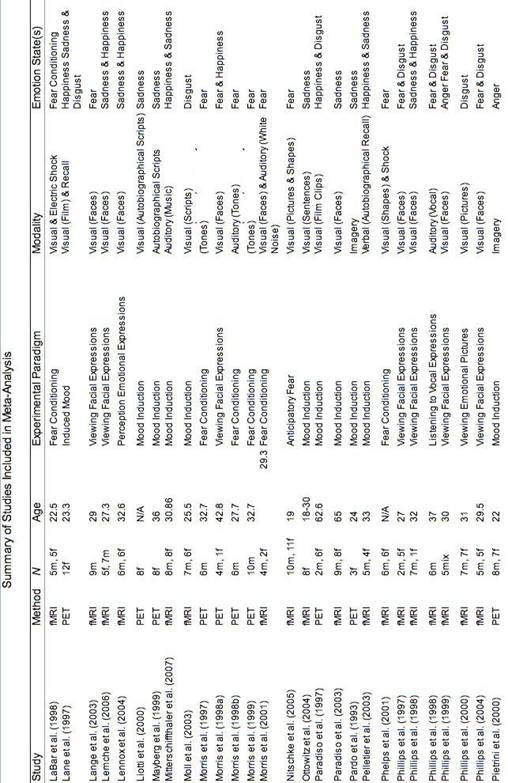

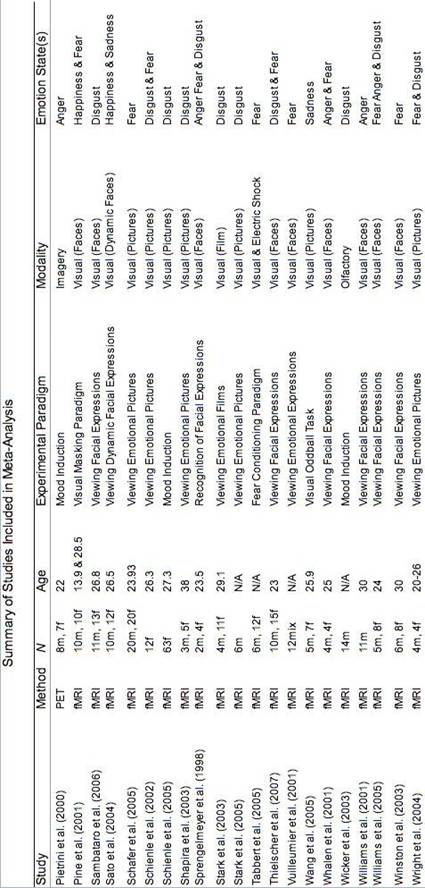

Table 2.
Figure 1. Main Effect of Happiness
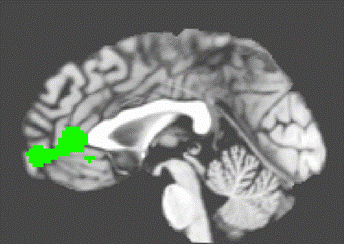
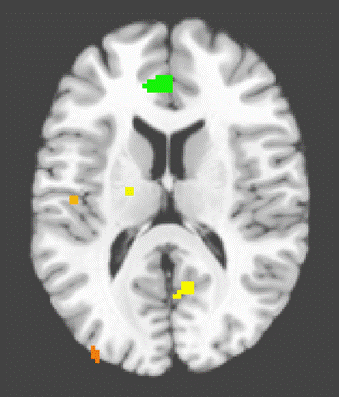
Anterior Cingulate (0,38,8)
Middle Frontal Gyrus (36,48,-8)
Insula
Thalamus (-18,-10,18)
Posterior Cingulate (10,-56,16)
Figure 5. Main Effect of Disgust
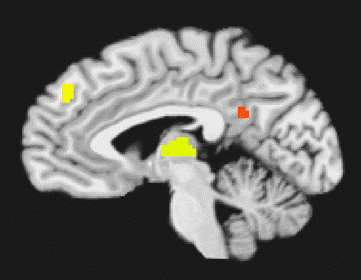
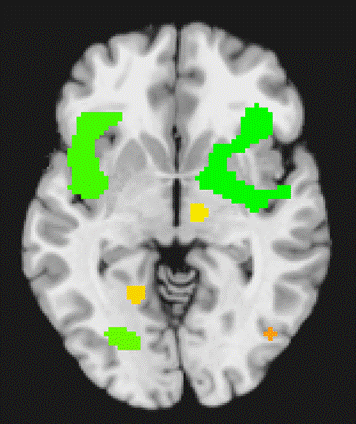
Inferior Frontal Gyrus
Medial Gyrus
ACC
Middle Temporal Gyrus
Although the term basic conveys with it evolutionary implications, it will be used interchangeably with discrete throughout this review in order to parallel the way it is used in the current psychological literature.
Murphy et al. (2003) defines affect programs as the (neural) mechanism that stores patterns for and triggers complex emotional responses that are often quick, complex, organized, and difficult to control.
Barrett (2006) examined both psychophysiological and neuroimaging data in a recent review, however, the data were not quantitatively analyzed in a meta-analysis.
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 2167
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2026 . All rights reserved