| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
Release Date: September 29, 2004; Valid for credit through September 29, 2005
CME in this activity indicates that it was developed according to ACCME
guidelines and was certified for credit by one or more accredited CME or CE
providers. Medscape cannot attest to the timeliness of expired CME activities.
This accredited activity has been developed for endocrinologists and diabetologists, as well as internists and primary care physicians with strong interest in the care of people with diabetes.
Many patients do not reach their target hemoglobin A1C levels, and the situation is not improving. To address this clinical challenge, new medications have been developed in an attempt to target the full spectrum of diabetes pathophysiology. Research into the use of incretins for diabetes treatment is opening a new therapeutic approach to clinicians seeking to restore normal glucose metabolic homeostasis. Such medications may soon be available. This activity will provide a comprehensive overview to familiarize clinicians with this emerging treatment approach, including the pathophysiology of diabetes, the pharmacologic rationale for use of incretins, and data on restoring incretin function in patients with diabetes.
Upon completion of this activity, participants will be able to:
All other healthcare professionals completing continuing education credit for this activity will be issued a certificate of participation.
Participants should claim only the number of hours actually spent in completing the educational activity.
For Physicians

The
The
![]()
For questions regarding the content of this activity, contact the accredited
provider for this CME/CE activity: cme@joslin.harvard.edu. For technical
assistance, contact CME@webmd.net.
There are no fees for participating in or receiving credit
for this online educational activity. For information on applicability and
acceptance of continuing education credit for this activity, please consult
your professional licensing board.
This activity is designed to be completed within the time designated on the
title page; physicians should claim only those credits that reflect the time
actually spent in the activity. To successfully earn credit, participants must
complete the activity online during the valid credit period that is noted on
the title page.
Follow these steps to earn CME/CE credit:
You may now view or print the certificate from your CME/CE
Tracker. You may print the certificate but you cannot alter it. Credits will be
tallied in your CME/CE Tracker and archived for 5 years; at any point within
this time period you can print out the tally as well as the certificates by
accessing 'Edit Your Profile' at the top of your Medscape homepage.
The credit that you receive is based on your user profile.
Supported by an unrestricted educational grant from Amylin
Pharmaceuticals, Inc. and Eli Lilly and Company.
![]()
Legal Disclaimer
The materials presented here do not necessarily reflect the views of Medscape,
The Joslin Diabetes Center, the companies providing educational grants or the
authors and writers. These materials may discuss uses and dosages for
therapeutic products that have not been approved by the United States Food and
Drug Administration. All readers and continuing education participants should
verify all information and consult a qualified healthcare professional before
treating patients or utilizing any therapeutic product discussed in this
educational activity.
This activity is offered in two formats. Please choose one of the versions:
Flash Version
(A multimedia presentation with synchronized audio, slides and transcript.)
Note: Macromedia's Flash Player 5 plug-in is required to view the Flash
version.
Download the Macromedia Flash Player 5 plug-In:
![]()
--OR--
Slides with transcript:
Diabetes and Intestinal Incretin Hormones: A New Therapeutic Paradigm
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD

Slide 1. Diabetes and Intestinal Incretin Hormones: A New Therapeutic Paradigm
Dr. Beaser: I am Dr. Richard Beaser, Medical Executive Director of
Professional Education at the
For those of us taking care of people with diabetes, it seems that we are always being challenged to do better. In recent years, studies have demonstrated the benefit of lower blood pressure, lower lipid levels, and -- at the core of diabetes management -- lower blood glucose levels.
Fortunately, we also have newer treatments to help us reach this goal. New classes of medications and insulin analogues have become available, allowing the restoration of near-normal insulin physiology and glucose homeostasis. We can now more precisely mimic natural insulin secretory patterns, and reduce both hepatic and peripheral insulin resistance.
Another step toward restoration of normal physiology is a treatment aimed at another component of the complex mechanism of insulin secretion and glucose metabolism. Incretin hormones -- the so-called 'gut hormones' -- have been recognized as playing a role in the coordination of insulin action with the influx of dietary carbohydrate. People with type 2 diabetes have a dysfunction in the action of these hormones. We are now on the verge of being able to replace the action of these hormonal coordinators, restoring yet another of the mechanisms needed to approach optimal glucose control.
In this program, we will explore how these hormones work, both in the normal state and in people who have type 2 diabetes. For those of us who provide medical care for people with diabetes, it is an opportunity to see what will soon be another therapeutic approach to treating this complex disease.
I would like to start by introducing our faculty for this program.
Dr. Bernard Zinman is Professor of Medicine at the
Dr. Edward Horton is Professor of Medicine at
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
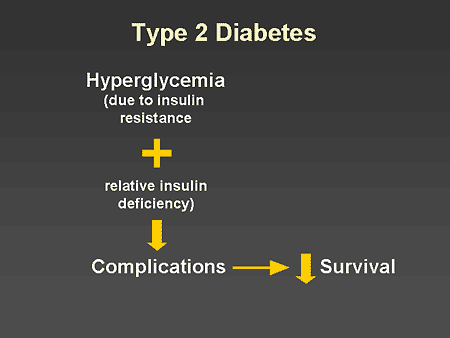
Slide 2. Type 2 Diabetes
Dr. Beaser: To understand the new treatments that will restore incretin function, it is important to start with a review of the pathophysiology of type 2 diabetes. I would like to ask Dr. Zinman to begin this discussion.
Dr. Zinman: Despite our best efforts, type 2 diabetes remains a chronic progressive metabolic disease characterized by hyperglycemia due to insulin resistance and relative insulin deficiency leading to end-organ complications and decreased survival.
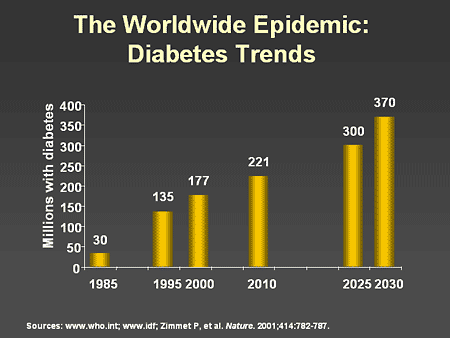
Slide 3. The Worldwide Epidemic: Diabetes Trends
Dr. Zinman: It is now well accepted that diabetes is a global problem and can be considered the most important chronic disease epidemic of the new millennium. In the year 2000, 177 million people around the world were known to have diabetes and this number will increase to over 350 million by the year 2030. The combined impact of a dramatic increase in rates of obesity, an increasingly sedentary lifestyle, and aging of the population, particularly in developed countries, are all contributing to this major health crisis.
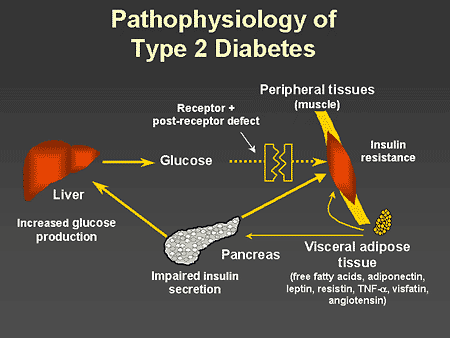
Slide 4. Pathophysiology of Type 2 Diabetes
Dr. Zinman: This slide illustrates the various pathways implicated in the pathophysiology of type 2 diabetes. It is now well-recognized that increased visceral adipose tissue plays a pivotal role in mediating the pathophysiologic events responsible for hyperglycemia and the other metabolic abnormalities that are associated with type 2 diabetes. Elevations in free fatty acids and changes in the concentration of adipokines appear to be responsible for mediating both insulin resistance and impaired insulin secretion. Of interest, adiponectin is unique in that high levels are associated with improved insulin sensitivity and improved beta-cell function. When visceral adipose tissue increases, adiponectin, unlike the other adipokines, decreases in concentration. The insulin resistance, characteristic of type 2 diabetes, is associated with increased hepatic glucose production and decreased insulin-mediated glucose transport at the muscle and adipose tissue. Impaired beta-cell function is also a prominent feature of the progressive nature of type 2 diabetes.
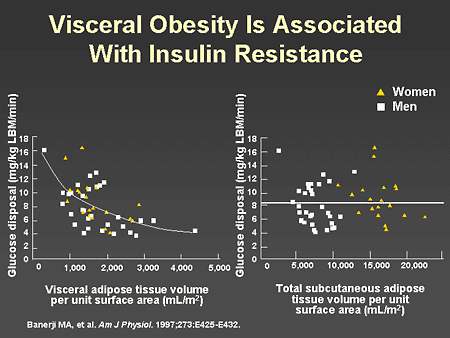
Slide 5. Visceral Obesity Is Associated With Insulin Resistance
Dr. Zinman: This slide illustrates the impact on glucose disposal of different volumes of visceral adipose tissue (left panel) compared with different volumes of subcutaneous adipose tissue (right panel), as assessed by abdominal computed tomography (CT) scan. Glucose disposal in response to an insulin infusion as part of the euglycemic glucose clamp is a sensitive indicator of insulin resistance. As seen in the left panel, glucose disposal is strongly related to the amount of visceral adipose tissue, with the highest rates of glucose disposal occurring when visceral adipose tissue volumes are low in both men and women. In contrast, as seen in the right panel, it is evident that despite large differences in subcutaneous adipose tissue volumes, glucose disposal remained constant. These data illustrate the importance of visceral fat in mediating the characteristic insulin resistance associated with type 2 diabetes. Let us now look more closely at beta-cell function.
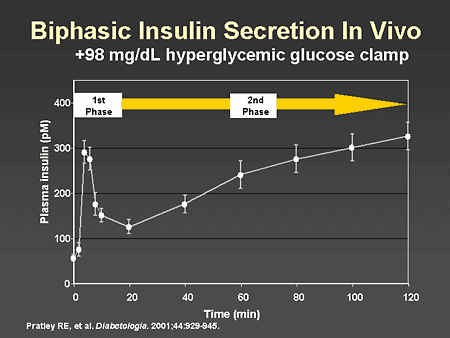
Slide 6. Biphasic Insulin Secretion In Vivo
Dr. Zinman: Insulin secretion in response to a standardized fixed glucose stimulus, as can be achieved with a hyperglycemic glucose clamp, has characteristically 2 components. First-phase rapid insulin release occurs within the first 5 to 10 minutes. This is followed by a more prolonged sustained insulin release, referred to as second-phase insulin secretion. In the context of type 2 diabetes, this normal physiology is disturbed early in the development of this metabolic abnormality. A prominent feature of type 2 diabetes is the loss of first-phase insulin secretion.
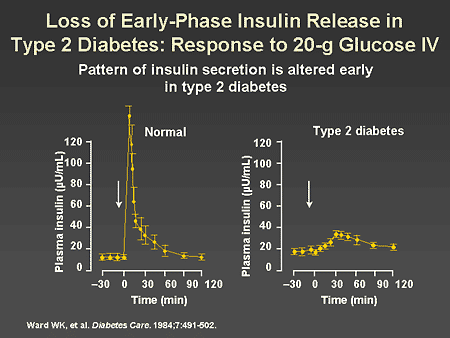
Slide 7. Loss of Early-Phase Insulin Release in Type 2 Diabetes: Response to 20-g Glucose IV
Dr. Zinman: This slide dramatically illustrates the pattern of altered insulin secretion in type 2 diabetes. In the left panel, we observe a brisk insulin secretory response to the intravenous injection of 20 g of glucose. As shown in the right panel, this response is completely lost in type 2 diabetes. Indeed, it is said that in type 2 diabetes, beta-cell function becomes essentially 'blind' to the stimulatory effects of glucose on beta-cell insulin release.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
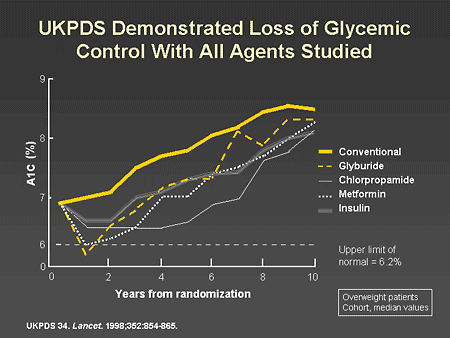
Slide 8. UKPDS Demonstrated Loss of Glycemic Control With All Agents Studied
Dr. Zinman: One of the important lessons learned from the United Kingdom Prospective Diabetes Study (UKPDS) is that type 2 diabetes is a progressive disease and despite the implementation of intensive therapy, A1C, which initially improved, continued to deteriorate over time. As illustrated on this slide, the use of glyburide, chlorpropamide, metformin, and even insulin in the UKPDS was associated with a progressive deterioration in A1C over time.
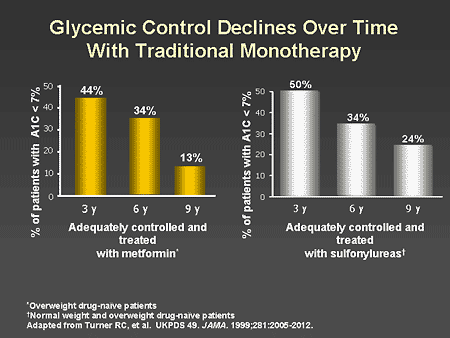
Slide 9. Glycemic Control Declines Over Time With Traditional Monotherapy
Dr. Zinman: Indeed as shown on this slide, the progressive nature of type 2 diabetes is manifest by a failure of traditional monotherapy to achieve clinical practice guideline targets of an A1C of less than 7%. The number of patients achieving an A1C of less than 7% declined progressively in those treated with both metformin (left panel) as well as those treated with sulfonylureas (right panel). It is important to note that after 3 years of therapy, approximately 50% of patients needed more than one oral antidiabetes agent.
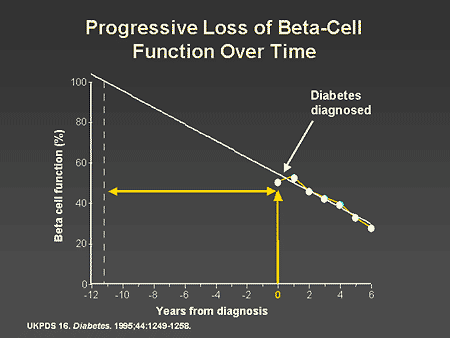
Slide 10. Progressive Loss of Beta-Cell Function Over Time
Dr. Zinman: It would appear that the deterioration in diabetes control observed in the UKPDS is a consequence of progressive loss of beta-cell function over time. As shown on this slide, at the time of diagnosis, 50% of beta-cell function had already been compromised and as the study continued, there was a further progressive decline in beta-cell function.
Dr. Beaser: Perhaps this is a good time to raise an interesting question about the loss of beta-cell function. We all assume that the reduction in function is due to a loss of beta-cell mass. Is this indeed the case, or are there other mechanisms, such as loss of signaling?
Dr. Zinman: Remarkably, despite long-duration type 2 diabetes, it would appear that a substantial amount of beta-cell mass may still be present and the problem is that beta-cell function is dramatically impaired.
Dr. Horton: I agree with Dr. Zinman that beta-cell function is impaired in patients with type 2 diabetes; however, data on beta-cell mass are somewhat controversial because beta-cell mass is very difficult to assess. The limited data we have are from autopsies, which suggest that there is also a loss of beta-cell mass, either through decrease of new beta-cell formation or an increase in beta-cell destruction by apoptosis.
Dr. Beaser: So what is the implication of this loss of beta-cell function?

Slide 11.
Dr. Zinman: As a result of the loss of beta-cell function, it is not surprising that over time, many patients with type 2 diabetes will need exogenous insulin therapy to control glucose.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD

Slide 12. The Importance of Glycemic Control
Dr. Beaser: Thank you, Dr. Zinman. We have heard for years that the goal of diabetes treatment is to achieve 'normal blood sugar.' But in reality, this is a complex concept with many clinical implications. Dr. Horton, please review for us your view of the goals of diabetes treatment.
Dr. Horton: It is now generally accepted that achieving good glycemic control in our patients with diabetes is extremely important to prevent both the microvascular and macrovascular complications and to improve overall quality of life. The American Diabetes Association (ADA), has established targets for pre- and postprandial glucose concentrations in day-to-day diabetes management and an A1C target of less than 7.0% as an indicator of long-term blood glucose control. The American Association of Clinical Endocrinologists in this country and the World Health Organization have set an even more rigorous A1C target of 6.5%. Numerous studies have shown that these targets are difficult to achieve and maintain with current therapies and that the majority of people with diabetes have not reached the established targets for good glucose control. This has led to a major effort to discover new and better treatments, some of which we will be discussing in this program. But first I would like to review the evidence on which our current targets for blood glucose control are based.
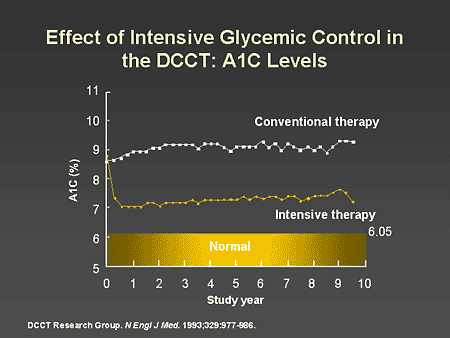
Slide 13. Effect of Intensive Glycemic Control in the DCCT: A1C Levels
Dr. Horton: There have now been a number of prospective studies that clearly demonstrate that improving blood glucose control and lowering A1C levels significantly reduces the risk of developing both microvascular and macrovascular complications. The Diabetes Control and Complications Trial (DCCT) compared a program of intensive diabetes management with one of conventional therapy in people with type 1 diabetes over a 9- to 10-year period. As shown on this slide, those receiving conventional therapy maintained an average A1C of approximately 9%, whereas those on intensive therapy were able to maintain an A1C of close to 7%, which is the current ADA A1C target.
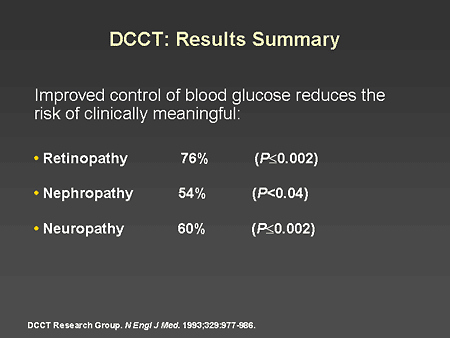
Slide 14. DCCT: Results Summary
Dr. Horton: This difference of approximately 2% in A1C level was associated with a 76% reduction in the development or progression of diabetic retinopathy, a 54% reduction in nephropathy, and a 60% reduction in clinically significant neuropathy.
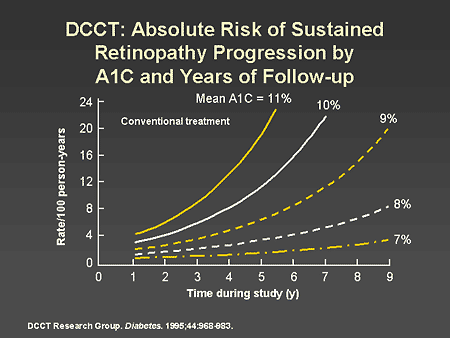
Slide 15. DCCT: Absolute Risk of Sustained Retinopathy Progression by A1C and Years of Follow-up
Dr. Horton: This slide shows the absolute risk of sustained retinopathy progression over time according to the mean A1C level in conventionally treated patients. Both the absolute risk and rate of progression of retinopathy were highest in those patients with high A1C levels, and very low when the A1C was maintained at 7%. Across the entire range of A1C levels, any improvement was associated with a decrease in risk. These data are the major rationale for the current ADA A1C target of 7% or less.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
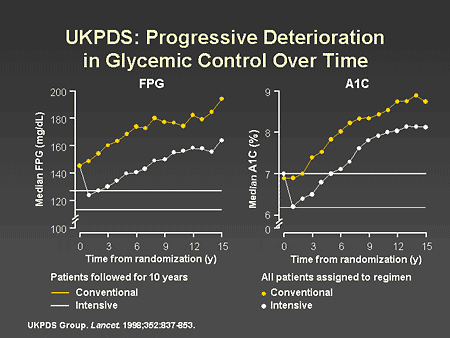
Slide 16. UKPDS Progressive Deterioration in Glycemic Control Over Time
Dr. Horton: In the UKPDS study of patients with type 2 diabetes, the intensively treated patients maintained fasting plasma glucose and A1C levels that were significantly lower throughout the study than those maintained by the conventionally treated patients. Even though both groups showed progressive worsening of glucose control over time, as has been previously discussed by Dr. Zinman, the average difference in A1C was maintained at approximately 0.9%.
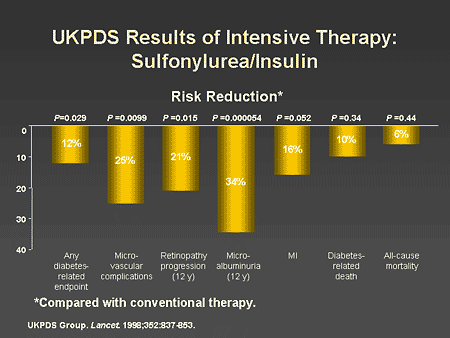
Slide 17. UKPDS Results of Intensive Therapy: Sulfonylurea/Insulin
Dr. Horton: This difference was associated with a 25% reduction in microvascular complications, particularly reduction in progression of retinopathy and development of microalbuminuria. There was also a borderline decrease in myocardial infarctions, but this did not reach statistical significance.
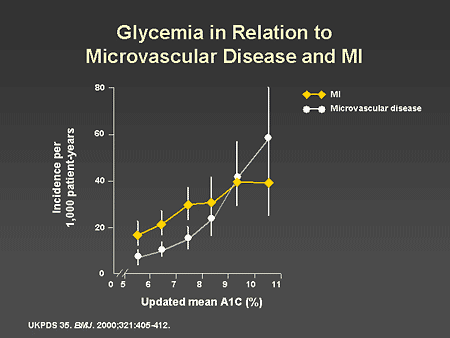
Slide 18. Glycemia in Relation to Microvascular Disease and MI
Dr. Horton: When all of the data from the UKPDS were pooled and analyzed as an epidemiologic study, there was a clear relationship between the average A1C level and the development of microvascular disease. This curve is very similar to the relationship between A1C and microvascular disease observed in the DCCT. There is also a significant relationship between A1C and myocardial infarction, though this is not as robust as the relationship of A1C to microvascular complications and undoubtedly reflects the multifactorial nature of atherosclerosis and coronary artery disease.
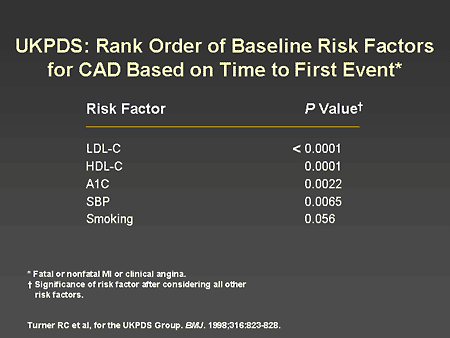
Slide 19. UKPDS: Rank Order of Baseline Risk Factors for CAD Based on Time to First Event
Dr. Horton: When multiple cardiovascular risk factors were analyzed in the UKPDS population, low-density lipoprotein (LDL) cholesterol turned out to be the most significant risk factor, and A1C level was third, ahead of systolic blood pressure and smoking.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
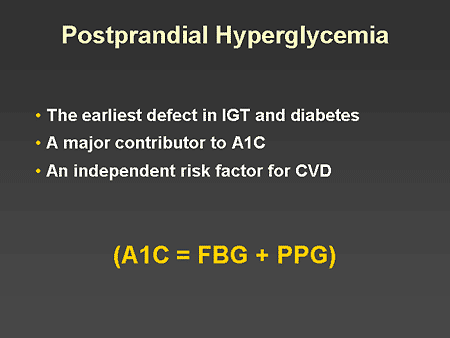
Slide 20. Postprandial Hyperglycemia
Dr. Horton: I would now like to turn our attention to the issue of treating postprandial hyperglycemia. In recent years there has been increasing awareness of the need to focus on the management of postprandial blood glucose levels in addition to treating fasting and preprandial glucose levels. There are several reasons for this. First, postprandial hyperglycemia is the earliest abnormality in patients with impaired glucose tolerance (IGT) or type 2 diabetes. Second, most of the day is spent in the postprandial state, and postprandial glucose levels are a major contributor to the A1C level. Finally, there is now abundant evidence that increased postprandial blood glucose is an independent risk factor for cardiovascular disease.
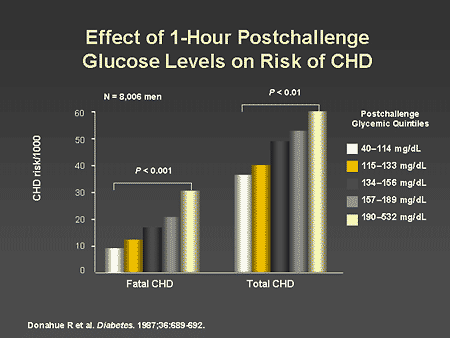
Slide 21. Effect of 1-Hour Postchallenge Glucose Levels on Risk of CHD
Dr. Horton: The relationship between impaired glucose tolerance and risk of coronary heart disease has now been demonstrated in several longitudinal studies. In the Honolulu Heart Study, there was a clear stepwise relationship between the 1-hour postglucose challenge blood glucose level and risk of fatal or nonfatal coronary heart disease. It is interesting to note that this relationship extends all the way from normal postchallenge levels to frank diabetes levels.
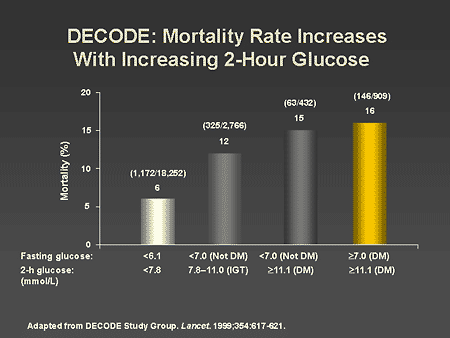
Slide 22. DECODE: Mortality Rate Increases With Increasing 2-Hour Glucose
Dr. Horton: The largest study relating postprandial hyperglycemia to
coronary heart disease mortality is the Diabetes Epidemiology: Collaborative
Analysis of Diagnostic Criteria in

Slide 23. Physiologic Determinants of Postprandial Hyperglycemia
Dr. Horton: I would like to conclude this section by discussing briefly the physiologic determinants of postprandial hyperglycemia as background for discussion of the potential role of incretins such as glucagon-like peptide-1 (GLP-1) and other related compounds in the treatment of type 2 diabetes.
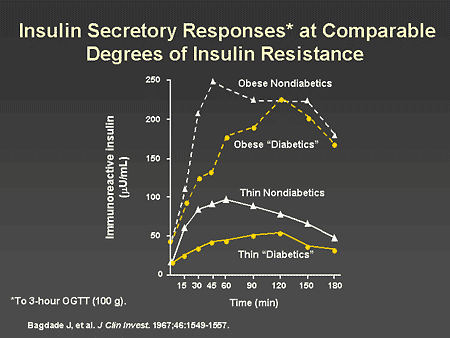
Slide 24. Insulin Secretory Responses at Comparable Degrees of Insulin Resistance
Dr. Horton: As has been discussed previously by Dr. Zinman, type 2 diabetes is associated with impairment of early insulin secretion. If one matches diabetic and nondiabetic patients for the degree of insulin resistance, you can see that the diabetic patient has a delay in the insulin secretory response to an oral glucose load compared with the nondiabetic individual.
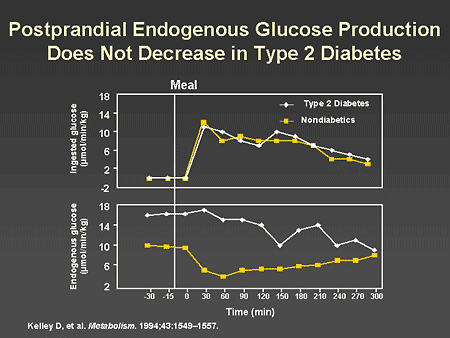
Slide 25. Postprandial Endogenous Glucose Production Does Not Decrease in Type 2 Diabetes
Dr. Horton: It is now clear that this lack of early insulin secretion contributes to postprandial hyperglycemia, primarily because of a failure to achieve rapid and effective suppression of hepatic glucose production. A study conducted by Dr. David Kelley and colleagues showed that absorption of ingested glucose is exactly the same in diabetic and nondiabetic subjects. However, people with diabetes have increased endogenous glucose production in the fasting state and fail to suppress it adequately in response to an oral glucose load. This is associated with a combination of insulin resistance in the liver and a decrease in the early, first-phase insulin secretory response, resulting in increased postprandial glucose levels.
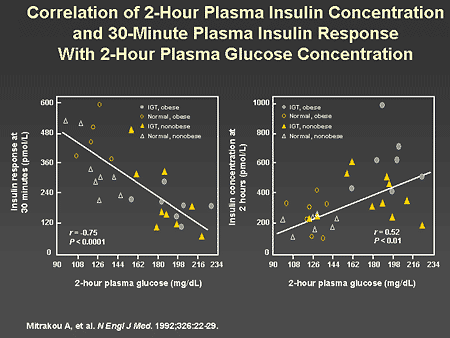
Slide 26. Correlation of 2-Hour Plasma Insulin Concentration and 30-Minute Plasma Insulin Response With 2-Hour Plasma Glucose Concentration
Dr. Horton: In another related study, correlations were made between the insulin response at 30 minutes and the 2-hour plasma glucose concentrations shown on the left, and the insulin concentration at 2 hours and the 2-hour plasma glucose shown on the right. There was an inverse correlation between the 30-minute insulin level and the 2-hour plasma glucose. In other words, the higher the early, first-phase insulin secretion, the lower the 2-hour glucose level. On the other hand, the insulin concentration achieved at 2 hours correlated positively with the 2-hour glucose value. This is interpreted to be a response to the sustained hyperglycemia.
Dr. Beaser: Dr. Horton, coming back to the issue of beta cells that we discussed earlier, can we preserve or restore beta-cell function in people with type 2 diabetes?
Dr. Horton: This has become the Holy Grail for finding new treatments, and there are many new and exciting developments in the field. It is clear from the UKPDS as well as from epidemiologic studies that currently available agents when used as monotherapy are not adequate to maintain good glycemic control over long periods of time. Combination therapies and a need for insulin replacement are the current standard of care. Although some data suggest that the thiazolidinediones and possibly other peroxisome proliferator-activated receptor (PPAR) gamma agonists may preserve beta-cell function to some extent, we do not yet have enough data to determine how effective they will be in the long run.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD

Slide 27. The Need for Global Treatment to Reduce the Risk of Complications
Dr. Beaser: This all comes back to that basic question: can we successfully reach treatment goals for people with type 2 diabetes, and thus reduce the risk of complications?
Dr. Horton: It is now clear that there are multiple risk factors for the development of atherosclerosis leading to macrovascular complications in patients with diabetes and that some of these risk factors, such as hypertension, also contribute to the development of microvascular disease. This means that we need to take a global approach to the treatment of patients with diabetes and focus on multiple risk factor reduction. This includes not only treatment of hyperglycemia but also aggressive treatment of hypertension and the dyslipidemia commonly seen in our patients, as well as attention to obesity, physical inactivity, and smoking.
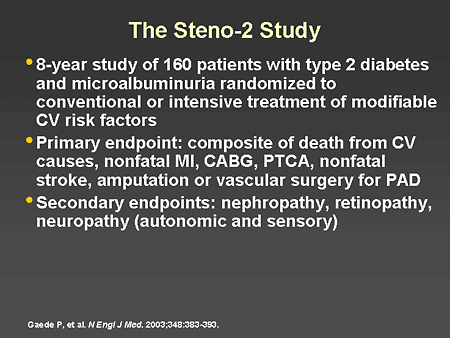
Slide 28. The Steno-2 Study
Dr. Horton: The effectiveness of this approach has been demonstrated
in the Steno-2 study (
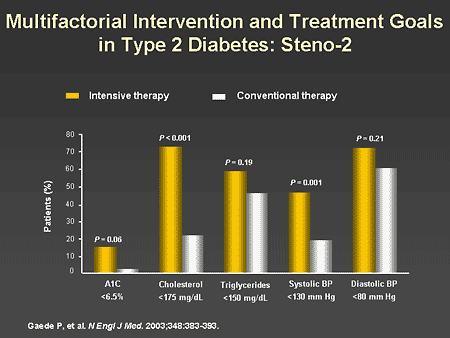
Slide 29. Multifactorial Intervention and Treatment Goals in Type 2 Diabetes: Steno-2
Dr. Horton: This slide shows the success rates in achieving the established targets for glucose, lipid and blood pressure control. As you can see, intensive treatment was significantly more effective than conventional therapy in achieving all of the targets except for diastolic blood pressure. The best results were in achieving total cholesterol levels less than 175 mg/dL, which was in 70% of patients, and the worst results were in achieving an A1C less than 6.5%, which occurred in only about 15% of patients.
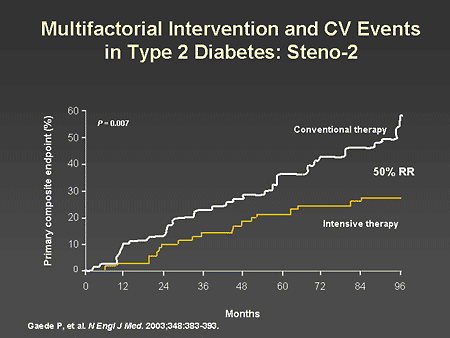
Slide 30. Multifactorial Intervention and CV Events in Type 2 Diabetes: Steno-2
Dr. Horton: The most striking finding of the study was a 50% reduction in risk for developing the primary composite endpoint of cardiovascular events.

Slide 31. Conclusion
Dr. Horton: This study clearly demonstrates the need for aggressive multifactorial risk reduction in patients with diabetes. But it is difficult to achieve our targets for glucose control with our currently available therapies, even in the setting of a major diabetes treatment center.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
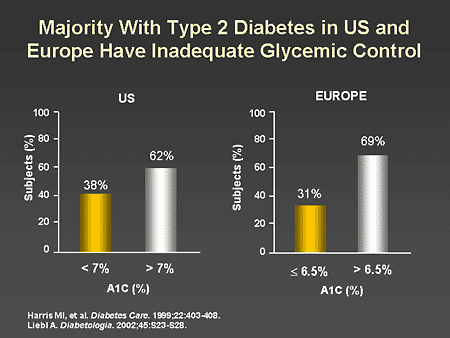
Slide 32. Majority With Type 2 Diabetes in US and
Dr. Beaser: Dr. Zinman, I understand this is not just a problem in
the
Dr. Zinman: Indeed, there is some additional evidence on the
difficulty in achieving glycemic targets in diabetes. As shown on this slide,
despite our best efforts, a majority of patients in the
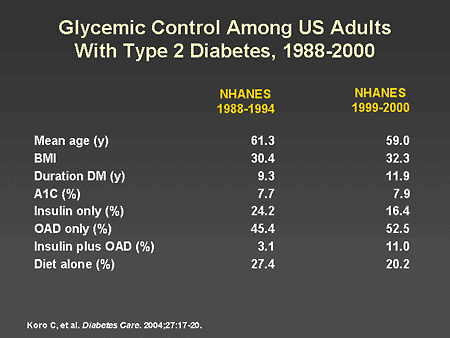
Slide 33. Glycemic Control Among US Adults With Type 2 Diabetes, 1988-2000
Dr. Zinman: The National Health and Nutrition Examination Survey
(NHANES) provides a population-based survey of nutritional health in the
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
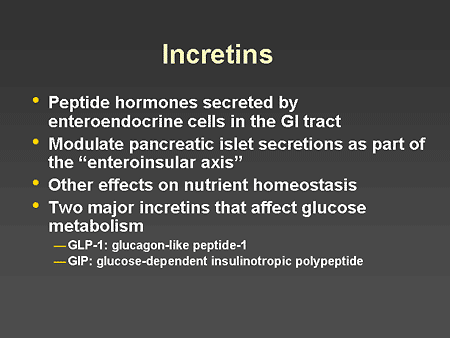
Slide 34. Incretins
Dr. Beaser: There is thus a clear need for newer diabetes treatments. One of the exciting developments is the therapies to restore incretin function. Dr. Horton, could you please describe these?
Dr. Horton: Incretins are peptide hormones that are secreted by the enteroendocrine cells in the gastrointestinal tract. Their major effect is to modulate pancreatic islet secretions as part of the so-called enteroinsular axis, although they also have been demonstrated to have other effects on nutrient homeostasis. The 2 major incretins that affect glucose metabolism are glucagon-like peptide-1, or GLP-1, and glucose-dependent insulinotropic polypeptide, or GIP.
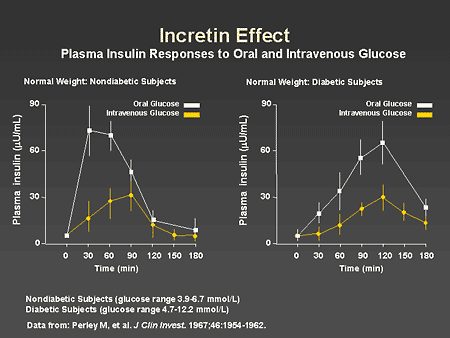
Slide 35. Incretin Effect: Plasma Insulin Responses to Oral and Intravenous Glucose
Dr. Horton: The incretin effect was described more than 35 years ago and refers to the fact that there is a much greater insulin response to glucose when it is given orally than when the same amount is administered as an intravenous infusion. This is illustrated on this slide, which shows the plasma insulin responses to oral and intravenous glucose in nondiabetic subjects, shown on the left, and in those with diabetes, shown on the right. In the normal weight nondiabetic subjects, you can see that the rise in plasma insulin after oral glucose administration is very rapid, reaching a peak concentration by 30 minutes and then falling off rapidly and returning to baseline by 3 hours. In comparison, the response to the same amount of glucose given intravenously is much lower and peak levels are not reached until approximately 90 minutes. In the diabetic subjects, shown on the right, oral glucose administration results in a much greater insulin response than intravenous glucose but in both circumstances there is a delay in reaching peak plasma insulin concentrations, which does not occur until approximately 2 hours.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
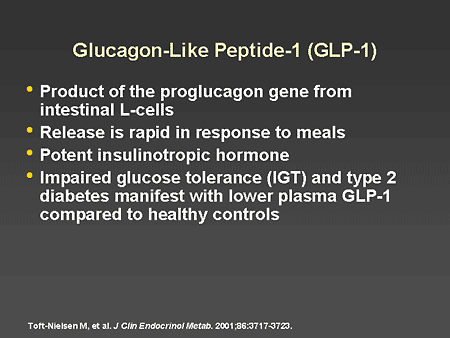
Slide 36. Glucagon-Like Peptide-1 (GLP-1)
Dr. Horton: It was not until the 1980s that GLP-1 and GIP were identified and their physiologic roles could be studied. Of the 2 incretins, GLP-1 is the more important one, although GIP is responsible for about 20% to 30% of the incretin effect on pancreatic beta-cell function. GLP-1 is a 37-amino acid polypeptide that is produced in intestinal L-cells as a part of proglucagon. It is released rapidly in response to the ingestion of glucose or a mixed meal and has very potent effects on pancreatic beta cells to increase insulin secretion in a glucose-dependent manner. It also suppresses glucagon secretion and has a number of effects that regulate the absorption and metabolism of nutrients. It is also important to note that people with IGT or type 2 diabetes have lower plasma GLP-1 levels in response to stimulation compared with healthy controls. This may contribute to the impaired beta-cell function seen in these conditions.
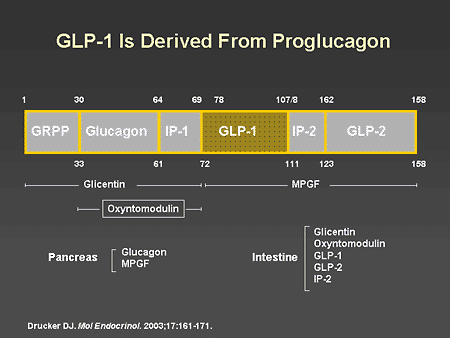
Slide 37. GLP-1 Is Derived From Proglucagon
Dr. Horton: As I mentioned, GLP-1 is a product of the proglucagon gene, which is expressed in both pancreatic islets and in intestinal L cells. In this schematic representation, you can see that proglucagon contains glucagon-related polypeptide (GRPP), GLP-1 and -2, and intestinal polypeptide 1 and 2. In response to a meal, GLP-1 is rapidly released as a 37-amino acid polypeptide or as a 36-amino acid polypeptide amide and rapidly reaches high concentrations in the intestinal mucosa and in the circulation.
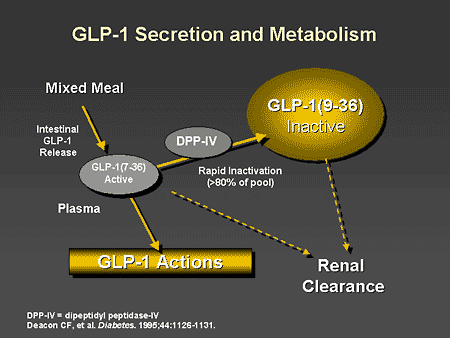
Slide 38. GLP-1 Secretion and Metabolism
Dr. Horton: As shown on this slide, the active form of GLP-1 is the 7-36 amino acid polypeptide, which binds to GLP-1 receptors on the beta cell and potentiates glucose-mediated insulin secretion as well as increased transcription of proinsulin and biosynthesis of insulin. Another very important feature of GLP-1 is that it is very rapidly inactivated by the ubiquitous enzyme dipeptidyl peptidase-IV, or DPP-IV, which cleaves 2 terminal amino acids converting the active 7-36 polypeptide to the inactive 9-36 polypeptide form. Both the active and inactive forms of GLP-1 are then cleared by the kidneys. Because of the rapid secretion and rapid inactivation of GLP-1, its biologic actions are very short lived and rapidly reversible.
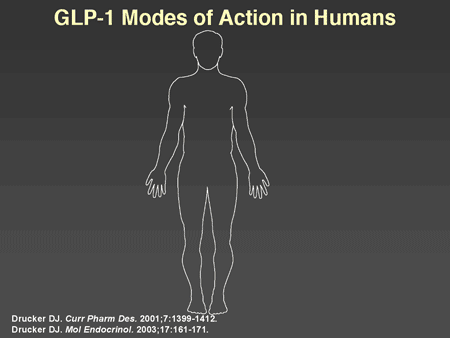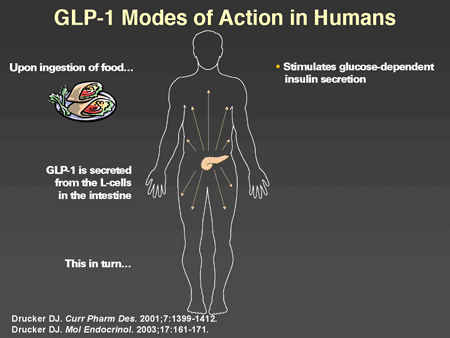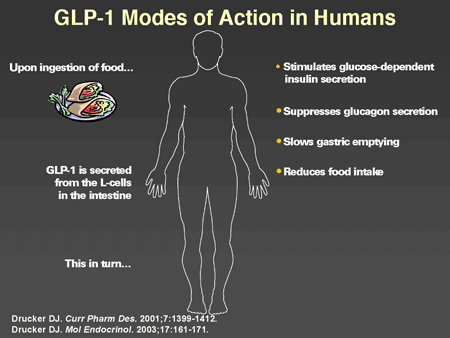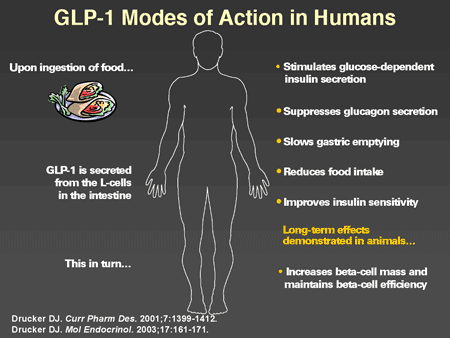
Slide 39. GLP-1 Modes of Action in Humans
Dr. Horton: GLP-1 has a number of actions in humans that have now been well characterized. When one ingests a mixed meal, GLP-1 is secreted from the intestinal L-cells, which in turn stimulates glucose-dependent insulin secretion. This is the originally described incretin effect. In addition, GLP-1 suppresses glucagon secretion, slows gastric emptying, and there are now data showing that it results in increased satiety and reduction of food intake. Originally there were some suggestions that it may directly improve insulin sensitivity but this is not well established. Its major biologic effects appear to be due to enhanced glucose-mediated insulin secretion and suppression of glucagon. Studies have been conducted in animal models that indicate that in addition to its acute effects, there are more chronic effects on beta cells to increase replication and neogenesis of new beta cells and to inhibit apoptosis. These findings are very encouraging and suggest that GLP-1 may play a significant role in the preservation and even possibly restoration of beta-cell mass and function in humans, although studies have not been done yet to confirm this.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
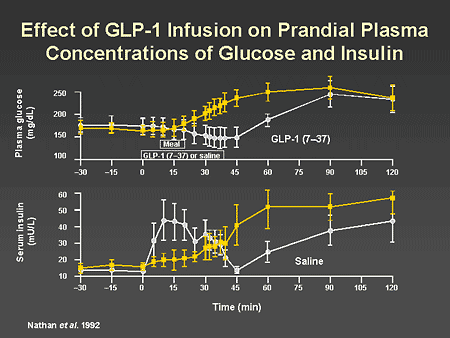
Slide 40. Effect of GLP-1 Infusion on Prandial Plasma Concentrations of Glucose and Insulin
Dr. Horton: There are now substantial data on the physiologic effects of GLP-1 in humans. This slide shows the effects of GLP-1 infusion on the plasma glucose and insulin responses to ingestion of a meal. In the lower panel you can see that compared with saline infusion, GLP-1 infusion results in a much greater insulin response, which reaches a peak very rapidly and then falls back to baseline after the infusion is discontinued. The corresponding glucose responses are shown in the upper panel. The GLP-1 infusion completely blunts the rise in plasma glucose in response to the meal but does not result in hypoglycemia.
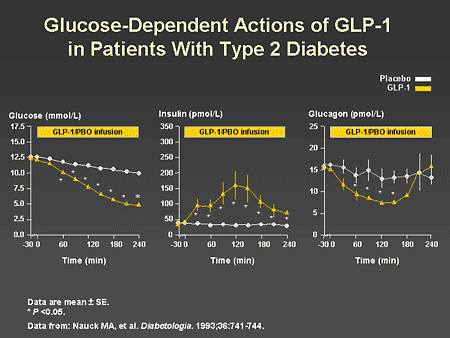
Slide 41. Glucose-Dependent Actions of GLP-1 in Patients With Type 2 Diabetes
Dr. Horton: Another important characteristic of GLP-1 is that its effects on insulin and glucagon secretion are dependent on glucose. If one infuses GLP-1 in people with type 2 diabetes, there is rapid increase in insulin and suppression of glucagon and a fall in glucose concentrations toward normal. However, as glucose levels fall into the normal or near-normal range, the effects on insulin and glucagon secretion are reversed, with insulin levels decreasing and glucagon increasing.
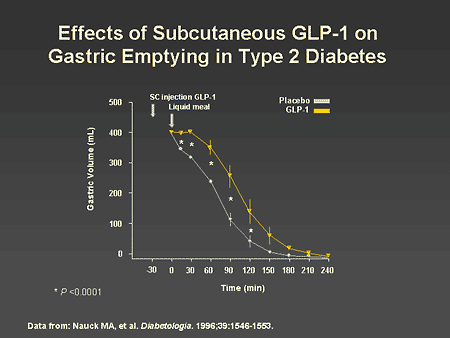
Slide 42. Effects of Subcutaneous GLP-1 on Gastric Emptying in Type 2 Diabetes
Dr. Horton: As mentioned earlier, another effect of GLP-1 is to delay gastric emptying. This is illustrated on this slide, which demonstrates that a subcutaneous injection of GLP-1 followed by ingestion of a liquid meal is associated with a 30-minute delay in gastric emptying, which is observed over a 2- to 3-hour period. The effect of this would be to delay the digestion and absorption of carbohydrates and thus decrease the postprandial rise in blood glucose levels. GLP-1 has also been demonstrated to result in increased satiety and decreased food intake. Some of this may be related to the delay in gastric emptying and afferent neural signals from the gastrointestinal tract to the central nervous system. However, there is also some evidence that GLP-1 may have direct effects on the central nervous system.
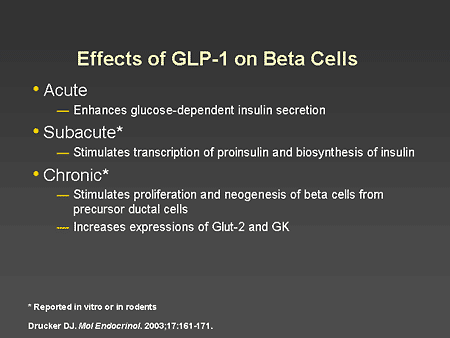
Slide 43. Effects of GLP-1 on Beta Cells
Dr. Horton: To summarize the effects of GLP-1 on beta cells, the best documented direct effects in humans are to enhance glucose-dependent insulin secretion. Subacute effects have been demonstrated in incubated human islet cells demonstrating that GLP-1 has major effects on glucose metabolism in beta cells and also increases the synthesis of insulin. Longer-term chronic effects have been observed in animal studies. These include increased proliferation and neogenesis of beta cells, decreased beta-cell apoptosis, and increased expression of important glucose-sensing factors such as Glut-2 glucose transporters and glucokinase.
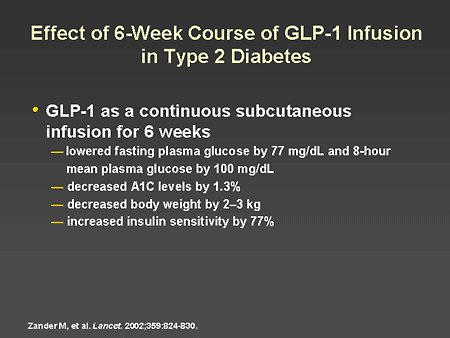
Slide 44. Effect of 6-Week Course of GLP-1 Infusion in Type 2 Diabetes
Dr. Horton: Dr. Zinman will be discussing, in much greater detail, the pathophysiology of incretins in people with diabetes and the clinical implications of therapy to restore incretin function. I would like to mention a study which I consider to be an important proof of principle that incretins will ultimately be useful for the treatment of people with type 2 diabetes. A study by Zander and colleagues evaluated the effects of a 6-week course of GLP-1 given by continuous subcutaneous infusion to patients with type 2 diabetes. They found that fasting plasma glucose was decreased by 77 mg/dL and 8-hour mean plasma glucose by 100 mg/dL. This was associated with a decrease in A1C of 1.3%, a decrease in body weight, and an improvement in insulin sensitivity. These are very encouraging findings and pave the way for the many newer studies that will be described by Dr. Zinman.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD

Slide 45. Strategies to Enhance Incretin Action in Diabetes
Dr. Beaser: We have discussed how these substances play a key role in glucose homeostasis. Next, let's superimpose type 2 diabetes. Dr. Zinman, how does incretin function, and dysfunction, contribute to this clinical picture?
Dr. Zinman: There are currently 3 strategies to enhance incretin action in patients with type 2 diabetes: 1) create GLP-1 analogues resistant to degradation by DPP-IV, with resultant prolonged pharmacokinetics; 2) administer exenatide, a synthetic formulation of Exendin 4, a natural component of salivary secretion from the Gila monster lizard, which is resistant to DPP-IV degradation and is a potent GLP-1 agonist; and 3) administer inhibitors of DPP-IV with the goal of inhibiting breakdown of endogenous GLP-1 secretion. GLP-1 analogues and exenatide are incretin mimetic agents.
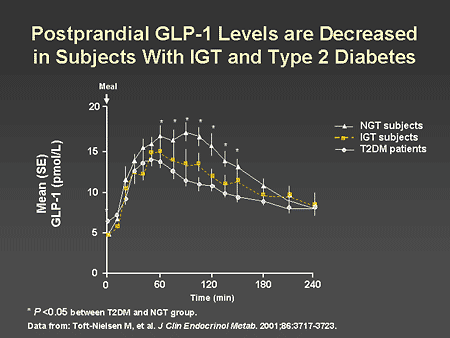
Slide 46. Postprandial GLP-1 Levels are Decreased in Subjects With IGT and Type 2 Diabetes
Dr. Zinman: As illustrated on this slide, it is important to recognize that GLP-1 secretion is abnormal in patients with IGT and type 2 diabetes. Compared with individuals with normal glucose tolerance, postprandial GLP-1 levels are significantly reduced both in patients with IGT and in patients with type 2 diabetes.
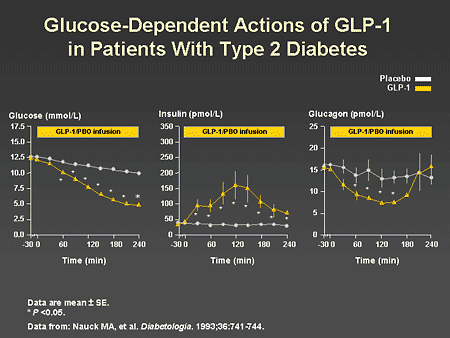
Slide 47. Glucose-Dependent Actions of GLP-1 in Patients With Type 2 Diabetes
Dr. Zinman: As previously reviewed by Dr. Horton, an important characteristic of GLP-1 is its glucose-dependent action on beta-cell secretion. As illustrated on the left panel of this slide, GLP-1 effectively lowers glucose to normal physiologic levels. This is a consequence of increased insulin secretion, as shown in the middle panel of this slide, and a reduction in glucagon, as shown in the right panel. Importantly, despite continued infusion of GLP-1, insulin secretion decreased rapidly when normal glycemia was achieved. Thus, inappropriate insulin secretion and hypoglycemia are avoided.
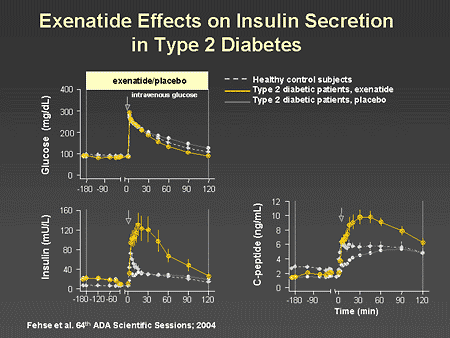
Slide 48. Exenatide Effects on Insulin Secretion in Type 2 Diabetes
Dr. Zinman: This slide illustrates the potent effect of exenatide on insulin secretion. In this study, the stimulus for insulin secretion is an intravenous glucose injection. As illustrated in the top panel, a similar glycemic stimulus was achieved in healthy controls, patients with type 2 diabetes given exenatide, and patients with type 2 diabetes given placebo. The middle and lower panels demonstrate that both insulin and C-peptide responses were abnormal in patients with type 2 diabetes treated with placebo, and a dramatic recovery occurred when exenatide was administered. This in essence represents a robust return of first-phase insulin secretion.
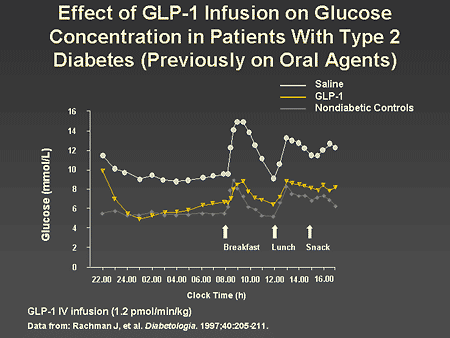
Slide 49. Effect of GLP-1 Infusion on Glucose Concentration in Patients With Type 2 Diabetes (Previously on Oral Agents)
Dr. Zinman: This slide demonstrates that in a more clinically meaningful setting, namely glycemic regulation during the night and in response to meals, GLP-1 infusion has a potent effect on normalizing overnight glucose control as well as the postprandial response to breakfast and lunch. As can be seen, compared with patients receiving a saline infusion, GLP-1 infusions were associated with a prompt and sustained lowering of glucose levels approaching those of the nondiabetic controls.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD

Slide 50. Objectives of Exenatide Pivotal Studies: Addition to Oral Agents
Dr. Zinman: At the 2004
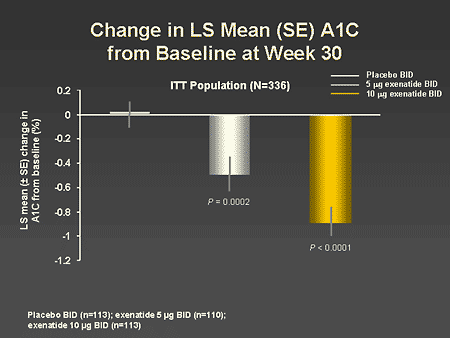
Slide 51. Change in Mean (SE) A1C From Baseline at Week 30
Dr. Zinman: As seen on this slide, the addition of exenatide to metformin therapy resulted in a dose-response improvement in A1C over a 30-week period.
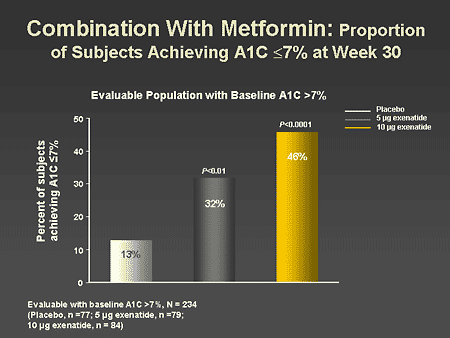
Slide 52. Combination With Metformin: Proportion Subjects Achieving A1C </= 7% at Week 30
Dr. Zinman: In another analysis of this therapeutic response, the
effect of adding exenatide to metformin resulted in a significant improvement
in the number of patients able to achieve an A1C of less than/equal to 7%.
Indeed, with 10 mcg of exenatide twice daily, 46% of patients achieved this
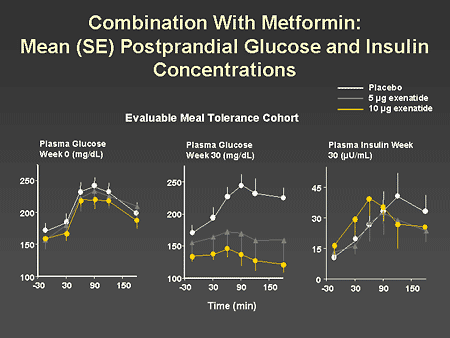
Slide 53. Combination With Metformin: Mean (SE) Postprandial Glucose and Insulin Concentrations
Dr. Zinman: This slide demonstrates the benefit of exenatide when added to metformin on postprandial glycemic control. In the left panel, we can see that the pretreatment plasma glucose response to a meal was similar for all treatment arms. After 30 weeks of therapy with either placebo, 5 mcg of exenatide twice daily, or 10 mcg of exenatide twice daily, significant changes in the glycemic profile associated with the meal were observed. It is important to note that both the premeal glucose and the postprandial response were markedly reduced in a dose-dependent manner with exenatide. In addition, in the right panel, one can see that the plasma insulin response with 10 mcg of exenatide was earlier and was consistent with a more physiologic insulin response to a mixed meal.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
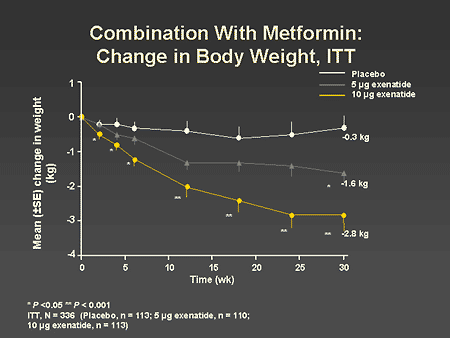
Slide 54. Combination With Metformin: Change in Body Weight, ITT
Dr. Zinman: The change in body weight over the 30-week treatment period is shown on this slide. A progressive dose-dependent reduction in body weight was seen with exenatide, and at 30 weeks, patients lost an average of 2.8 kg with the 10-mcg twice-daily dose. In this context, it is important to note that the side effect of nausea occurred early after the initiation of therapy and the weight loss persisted long after the symptoms of nausea had evolved.
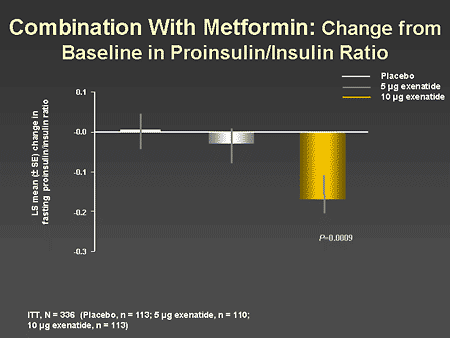
Slide 55. Combination With Metformin: Change From Baseline in Proinsulin/Insulin Ratio
Dr. Zinman: The proinsulin/insulin ratio is considered to be an objective measure of beta-cell function. High ratios of proinsulin/insulin suggest beta-cell stress reflecting a defect in insulin processing. A reduction in the proinsulin/insulin ratio is a marker of improved beta-cell health. As is evident from this slide, there was a significant reduction in the proinsulin/insulin ratio with the 10-mcg dose of exenatide.
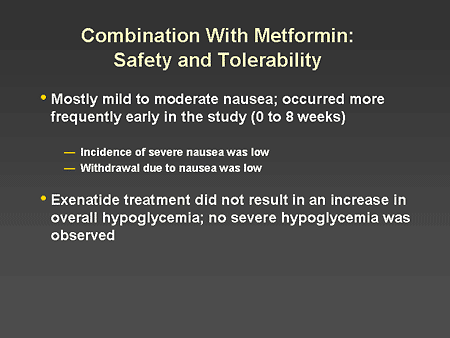
Slide 56. Combination With Metformin: Safety and Tolerability
Dr. Zinman: The only significant adverse effect of combining exenatide with metformin was an increase in mild to moderate nausea. This occurred more frequently early in the study and tended to disappear after 8 weeks of treatment. The incidence of severe nausea was low: 2% in placebo, 3% in the 5-mcg exenatide group, and 4% in the 10-mcg exenatide group. Withdrawal due to nausea was low, occurring in 1% in the 5-mcg exenatide group and 3% in the 10-mcg exenatide group. When used with metformin, exenatide treatment did not result in any increase in overall hypoglycemia, and no severe hypoglycemia was observed.
Slide 57. Similar Results With Exenatide and Other Oral Antidiabetes Agents
Dr. Zinman: As part of the exenatide/oral antidiabetes combination therapy program, long-term studies of exenatide with sulfonylureas and exenatide with combined metformin/sulfonylureas have been completed. These results are very similar to the data just presented for exenatide in combination with metformin. The only observed difference relates to the increase in mild to moderate hypoglycemia in those patients taking a sulfonylurea.

Slide 58. Summary
Dr. Zinman: In summary, exenatide treatment for 30 weeks in patients with type 2 diabetes who are unable to achieve glycemic control with maximum effective doses of metformin, sulfonylureas, or metformin/sulfonylurea combinations resulted in a significant dose-dependent reduction in fasting glucose, A1C, body weight, reductions in postprandial glucose, and improvements in beta-cell function. The most frequent adverse events were gastrointestinal in nature, generally mild to moderate, and occurred more frequently at initiation. Patients on sulfonylureas had increased mild to moderate hypoglycemia.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
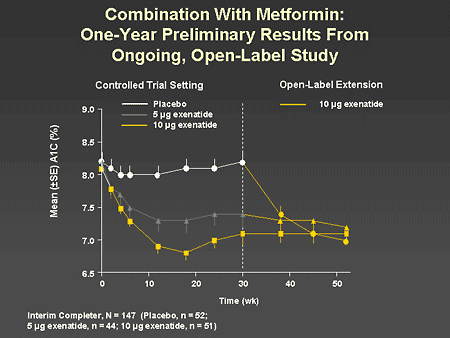
Slide 59. Combination With Metformin: One-Year Preliminary Results From Ongoing, Open-Label Study
Dr. Zinman: This slide shows the response to the combination of exenatide with metformin during the 1-year open-label extension. Those patients initially randomized to placebo and then treated with exenatide in the open-label extension had a prompt reduction in A1C equivalent to the 2 active-treatment arms. It is also important to note that the reductions in A1C appeared to be sustained over the entire period of observation.
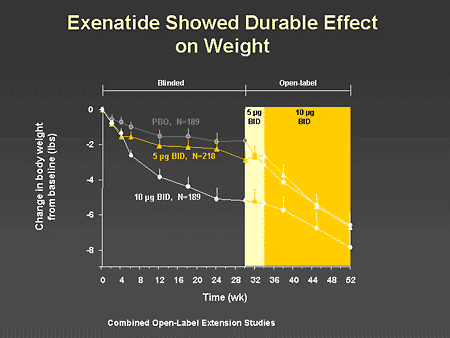
Slide 60. Exenatide Showed Durable Effect on Weight
Dr. Zinman: Remarkably, the open-label extension studies with exenatide and oral antidiabetes agents demonstrate a progressive and persistent weight loss over the year of follow up.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD

Slide 61. Strategies to Develop Long-Acting Incretin Mimetics
Dr. Zinman: The pharmacokinetic properties of exenatide require that it be administered by subcutaneous injection twice daily. Not surprisingly, there is intense activity to develop long-acting incretin mimetics. A long-acting, slow-release exenatide (LAR) is being developed that potentially could be administered once weekly or perhaps even once monthly. Another strategy is to develop GLP-1 agents that bind to circulating albumin and thus, have an extended activity. In addition, GLP-1 agents bound to large molecules that extend their clearance are also being tested. The development of long-acting GLP-1 agents will have 2 important beneficial effects. First, it will prolong the biologic action of these compounds, and second, it will enhance patient compliance with this form of therapy.
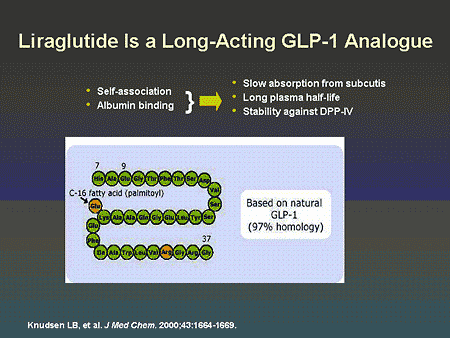
Slide 62. Liraglutide Is a Long-Acting GLP-1 Analogue
Dr. Zinman: An example of a long-acting GLP-1 analogue, liraglutide, is shown on this slide. The improved pharmacokinetics are a consequence of self-association and binding to albumin. This results in a prolonged plasma half-life due to resistance to DPP-IV degradation.
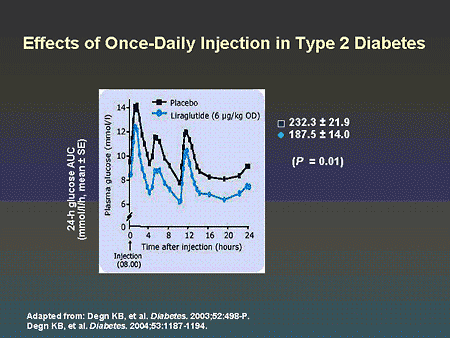
Slide 63. Effect of Once-Daily Injection in Type 2 Diabetes
Dr. Zinman: As shown on this slide, once-daily injection of liraglutide has a 24-hour effect on glycemic control in patients with type 2 diabetes.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD
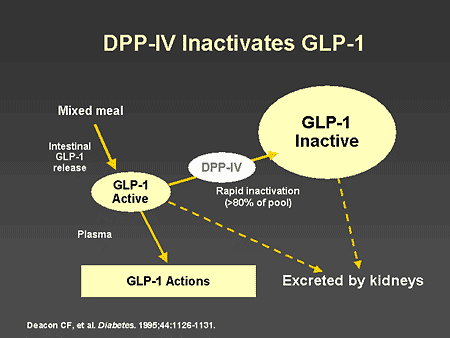
Slide 64. DPP-IV Inactivates GLP-1
Dr. Zinman: This slide schematically illustrates the fate of endogenous GLP-1 released from the intestinal L-cells in response to a mixed meal. As can be seen, DPP-IV rapidly inactivates GLP-1, and the inactive peptide is excreted by the kidneys.
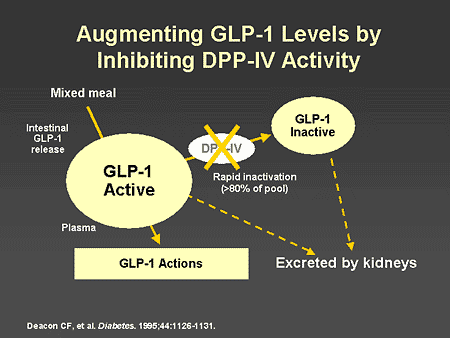
Slide 65. Augmenting GLP-1 Levels by Inhibiting DPP-IV Activity
Dr. Zinman: Given this physiology, a strategy designed to increase endogenous GLP-1 activity has focused on the development of compounds that would inhibit DPP-IV activity and thus increase endogenous GLP-1 action.
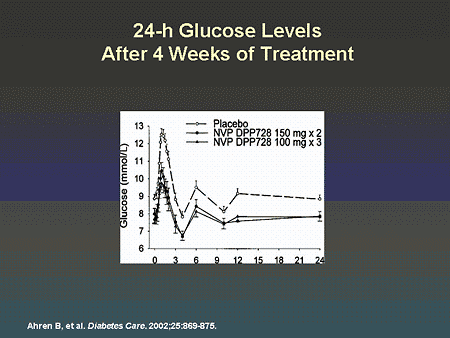
Slide 66. 24-h Glucose Levels After 4 Weeks of Treatment
Dr. Zinman: Indeed, studies have shown that metabolic control can be improved in patients with type 2 diabetes receiving a DPP-IV inhibitor. As illustrated on this slide, significant improvement in the glucose profile was seen with 2 dose levels of this DPP-IV inhibitor. Longer-term studies with the same compound have shown sustained and clinically significant improvements in A1C similar to that observed with exenatide.
Richard S. Beaser, MD; Edward S. Horton, MD; Bernard Zinman, MD

Slide 67. Use of Incretin Mimetic Agents and DPP-IV Inhibitors and the Management of Type 2 Diabetes
Dr. Zinman: In summary, increasing incretin action as a therapy for the management of type 2 diabetes appears to have a significant impact on diabetes control and may represent a paradigm shift in the management of this common metabolic disorder.
Dr. Beaser: Clearly these new classes of medications hold promise for improving diabetes control.
In our quest to restore normal glucose homeostasis and physiologic insulin action, we are on the verge of taking another important step forward. The treatments to restore incretin function will allow us to address yet another component in the complex cascade of events that normally support proper glucose metabolism for people with type 2 diabetes.
Our objective was to introduce the concept of incretin-focused therapies, and explore why this approach holds such promise for improving the control of people with diabetes.
On behalf of the Joslin Diabetes Center Professional Education Department, I am Dr. Richard Beaser, thanking you for participating in this continuing medical education program.
Flash Section
It is the policy of
If a faculty member has no information to disclose or refuses to do so, this information will also be provided.
|
Assistant Clinical Professor of Medicine, |
|
Professor of Medicine, |
|
Professor of Medicine, |
|
Registration for CME credit, the post test and the
evaluation must be completed online. |
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 3604
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2025 . All rights reserved