| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
DOCUMENTE SIMILARE |
||||
|
||||
TERMENI importanti pentru acest document |
||||
| : | ||||
Fern Spore Bank at the Royal Botanic Gardens
The aim of
this project was to determine the viability of spores held in the fern spore
bank in the Botanical Gardens in
Introduction
With over 12,000 species, ferns (Pteridophyta) are the second largest group of vascular plants on the planet, and are keystone species in some ecosystems. The trunk of Dicksonia antartica, a tree fern in Tasmania, supports a large variety of epiphytes, many of which grow exclusively on it (Smith et al, 2004), while other keystone fern species support a wide range of invertebrates (Ellwood et al, 2002).They also provide secondary compounds (Soeder, 1985) of pharmaceutical and agronomical interest. One of the species tested in this project, Dryopteris filix-mas was once used as an anthelmintic to expel intestinal worms (Waller et al, 2001). A highly proliferous species, Azolla, lives in symbiosis with cyanobacteria and fixes nitrogen, and so is used in Asian paddy fields as a cheap and natural alternative to chemical fertiliser. Azolla can be used as medicine, animal feed, water purification, biogas and hydrogen fuel production, and to control weeds and mosquitoes (Wagner, 1997). Given these properties, it is a matter of concern that by 2007, 193 species of ferns were listed on the IUCN Red List of Threatened Species (IUCN, 2007).
The RBGE, as part of a worldwide initiative, is therefore developing an ex situ spore bank to save endangered fern species from extinctions caused by insufficient variation within the genetic pool of isolated populations. Along with in situ fern conservation, this will provide support to conservation projects by using the banked spores to propagate those species in places where native populations have been fragmented by large-scale clearances of vegetation (Thomas et al, 2004). Large quantities of spores are easily collected to give a representative sample of a populations diversity, and take little space to store, thus allowing a comprehensive spore bank to be established; gametophytes would have been harder to obtain, are short-lived, and have to be fertilised to give new sporophytes (see figure 1) (Dyer ,1994).
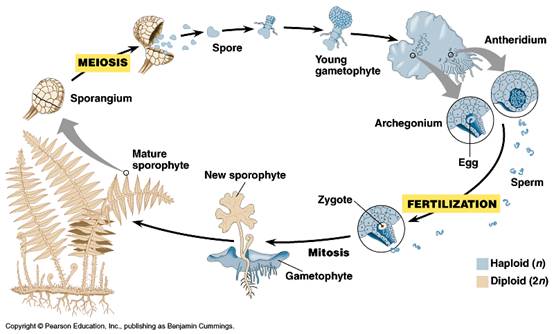
Fig. 1: Diagram showing the various stages of a Ferns lifecycle io.uwinnipeg.ca, 2005
Available storage techniques are being tested on different species of ferns to determine how they affect their ability to germinate (see RBGE website). Correct storage conditions are critical to preserve the spore collection that is difficult to restock for rare, endangered species.
Replenishing these stocks by harvesting spores from the Gardens own ferns more than three times is not permitted by the RBGE, so as to prevent any adaptation of the ferns to the particular conditions of the Garden (McHaffie, 2008).
The spores can be stored in closed vials (dry
storage) or on agar (wet storage) at freezing temperatures (
The RBGE provided spores from five different species to test their viability. Of particular interest were the spores of the rare Woodsia ilvensis. Storage conditions had varied and some spores had been subjected to temperature fluctuations.
 Dryopteris affinis: Native to southern and western
Dryopteris affinis: Native to southern and western
Fig. (www.mygarden.me.uk/Dryopteris%20affinis.jpg, 2008)

Dryopteris filix-mas: Native to the northern hemisphere. Sample collected in 1987. Spores were stored in freezer at -20oC.
Fig. (www.mygarden.me.uk/Dryopteris%20filix-mas.jpg, 2008)

Athyrium filix-femina: Native to the northern hemisphere. Sample collected in 1986. Spores were stored in freezer at -20oC.
Fig. (www.mygarden.me.uk/Dryopteris%20affinis.jpg, 2008)
 Todea barbara:
Native to Africa,
Todea barbara:
Native to Africa,
Fig. (www.apstas.com/blechnumnud.jpg, 2008)

Woodsia ilvensis: is a rare fern, it has wintergreen leaves that are between 4 and 20cm long. (Fitter et al, 1984) Spores were collected in 2002 from different sites including Feshie, Clova, Wasdale and Moffat site 2.
Fig. (www.ukbap.org.uk/UKPlans, 2008)
Woodsia ilvensis
Woodsia ilvensis is classified as endangered, and is
protected under Schedule 8 of the Wildlife and Countryside Act 1981 (t is a small fern found on rocky outcrops above 350m.
Currently there are less than 100 plants remaining in
The reasons of its decline are
unclear but climate change and over-collection during the 19th
century are thought to be factors responsible for the diminishing population in
As well as holding the British conservation collection of Woodsia ilvensis that represents most of the genetic variation of the population, the RBGE also regularly monitors the wild plants. A local conservation collection is maintained, grown from spores taken from the few remaining wild-growing plants (under licence). Some of these plants have been used for re-introductions into areas where this fern once grew.
Method
Medium preparation:
51 g/dm3 of magnesium sulphate,
12 g/dm3 of potassium nitrate,
1.7 g/dm3 of ferric chloride hydrate,
144 g/dm3of calcium chloride and
25 g/dm3 of potassium di-hydrogen orthophosphate were added to
1 ml/dm3 of nystatin (fungicide) was added to the solution to reduce fungal infection.
The final solution was sterilised in an autoclave, then poured into small Petri dishes.
Preparation of spores:
Fern spores were separated from the fronds and sori and put into test tubes on 13 February, 2008. (see figure 2 below).

Fig:2 Single sorus on leaf.
Sowing:
The openings of the test tubes were covered with 4 layers of lens tissue to filter sporangial debris and obtain an even spore density in all the Petri dishes.
The spores were sown by firmly tapping the inverted test tubes over the Petri dishes. The tubes were given either three taps to give densities of 1000 spores/cm2 (low), or ten taps to give densities of 3000 spores/cm2 (high). (see figure 3 below).
The Petri dishes were placed on a North-facing
interior windowsill. Using electric lamps, the ambient temperature was
maintained at a consistent
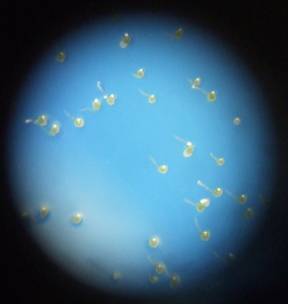
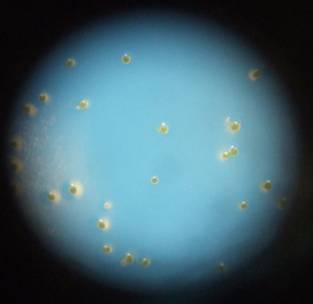
Fig:3 Todea barbara spores sown at high (left) and low (right) densities. Note germination on high density plate.
Counting:
Counting procedures were performed on 26 February, 3 March and 6 March, 2008.
The plates were taken out and straight lines were drawn at random with a marker pen on the underside of each plate to guide counting. (see figure 4 below)
Using a dissecting microscope at 45 magnification, germination was assessed by counting all spores lying along the lines until 100 spores were counted.
A spore was considered to have germinated when a rhizoid could be seen.
The number of spores germinated among the 100 counted was considered to be an estimate of the total percentage of germination. Counting was repeated five times for each plate in order to get the average percentage of germination.

Fig:4 Note lines for counting on bottom of dish. As you can see even distribution of the
spores was not achieved. This later interfered with counting (see evaluation)
Results
|
Days after sowing: |
10 days |
15 days |
18 days |
|
Woodsia ilvensis Clova (test sowing) |
N/A |
||
|
Woodsia ilvensis Wasdale (test sowing) |
N/A |
||
|
Woodsia ilvensis Moffat (test sowing) | |||
|
Woodsia ilvensis Feshie (test sowing) | |||
|
Dryopteris affinis 1970 (test sowing) | |||
|
Dryopteris affinis 1990 (test sowing) |
N/A |
N/A |
|
|
Dryopteris filix-mas 1987 (test sowing) | |||
|
Athyrium filix-femina 1986 (test sowing) |
N/A |
N/A |
|
|
Days after sowing: |
8 days |
13 days |
16 days |
|
Woodsia ilvensis Clova (high density) |
N/A |
||
|
Woodsia ilvensis Clova (low density) |
N/A |
||
|
Woodsia ilvensis Wasdale (high density) |
N/A |
||
|
Woodsia ilvensis Wasdale (low density) |
N/A |
||
|
Woodsia ilvensis Moffat (high density) | |||
|
Woodsia ilvensis Moffat (low density) |
N/A |
||
|
Woodsia ilvensis Feshie (high density) | |||
|
Woodsia ilvensis Feshie (low density) | |||
|
Dryopteris affinis 1970 (high density) | |||
|
Dryopteris affinis 1970 (low density) | |||
|
Dryopteris affinis 1990 (high density) |
N/A |
||
|
Dryopteris affinis 1990 (low density) |
N/A |
||
|
Dryopteris filix-mas 1987 (high density) |
N/A | ||
|
Dryopteris filix-mas 1987 (low density) |
N/A | ||
|
Athyrium filix-femina 1986 (high denisty) |
N/A |
N/A |
|
|
Athyrium filix-femina 1986 (low density) |
N/A |
N/A |
|
|
Todea barbara (high density) |
N/A |
N/A |
|
|
Todea barbara (low density) |
N/A |
N/A |
![]()
|
Woodsia ilvensis | |||||
|
Clova (6 years) |
Wasdale (6 years) |
Feshie (8 years) |
Moffat (8 years) | ||
|
Average germination |
<1% | ||||
|
First observed germination (days after sowing) |
10 days |
13 days |
10 days |
15 days | |
![]()
|
Dryopteris affinis |
||
|
Age of spores |
(18 years) |
(38 years) |
|
Average germination |
<1% |
|
|
First observed germination (days after sowing) |
8 days |
18 days |
|
Dryopteris filix-mas (20 years) |
Athyrium filix-femina (22 years) |
|
|
Average germination | ||
|
First observed germination (days after sowing) |
16 days |
8 days |
Evaluation of experiment
1. It was impossible to produce consistent spore densities due to eddies in the surrounding air at the moment of sowing on the Petri dishes that blew the tiny spores around. The difference between low and high densities, however, was markedly visible under the microscope.
2. Fungal and bacterial infections were present in almost all the Petri dishes. The extent of infection was minor at the time of counting; therefore it is assumed that the principal factors affecting germination were the length and method of storage (see figure 5 below).
3. Temperature may not have been consistent as dishes were stored in two rows on the windowsill, one by the window and the other nearer the lamps. However, because the dishes were returned in random order after each counting, the effects of this variation may be minimal.
4. Counting procedures were not carried out on March 19, when the final photographs of the dishes were taken, as the presence of visible gametophytes prevented accurate counting. Firstly, the gametophytes were generally found to be growing on just one side of the dish, probably because of air disturbance during sowing which caused the spores land unevenly on that side of the plate, so any counting would have had an inherent bias depending on the location chosen for counting. Secondly, the gametophytes were either hiding other germinated and non-germinated spores, or it was impossible to distinguish their extensive rhizoids from the rhizoids of other spores.
5. The absence of control spores affected our ability to interpret the results accurately.
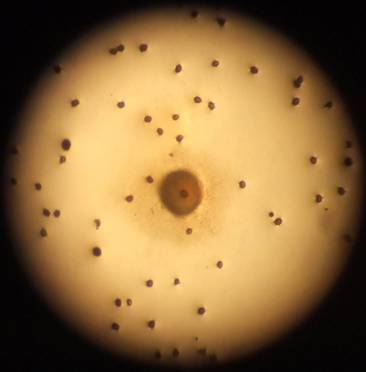
Fig:5 Bacterial infection surrounding spore in centre.
Discussion
From the results obtained, it appears that fern spores require higher densities (possibly for hormonal reasons) in order to increase the rate and percentage of germination. Woodsia ilvensis spores may be stored for up to 6, and possibly 7 years, if the germination rate of 26% or lower is sufficient to meet the purposes of the RBGEs conservation program. Storing them for over 8 years seems to greatly lower the germination rate of these spores down to ~ 1% or less (see figure 6)
Long-term storage of Dryopteris affinis spores appears to have had no major effects on germination. After 18 years of storage, approximately 85% of the spores germinated. However, of the spores stored for 38 years of storage, none germinated. An upper time limit for the storage of D. affinis spores could not be accurately determined, due to large variations in the ages of the spore samples.
The spores of Dryopteris filix-mas (stored 20 years) and Athyrium filix-femina (stored 22 years) were significantly affected by long-term storage which resulted in low germination rates of 2% and 8% respectively. Thus 20 to 22 years may represent the upper limits of time that these two ferns can be stored in this way.
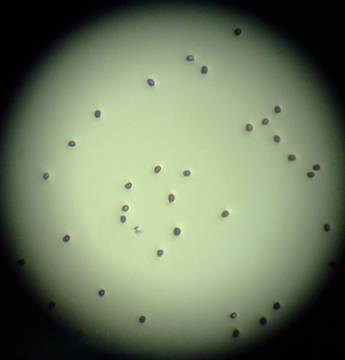
Fig:6 no germination on spores from Moffat after 8 years in storage
Points of Interest
Often the spores are sterilised prior to storage, this may have an effect if symbiants are necessary for germination.
This was a very small study, but by disruption of delicate sites to collect individual plants to retrieve spores this could seriously jeopardise vulnerable populations, unfortunately this is a necessity.
Other techniques of recovering lost species are
being researched because spores appear to survive for a very long time in their
natural environment and can be retrieved, still viable, from as far down in the
soil as
If the spores are stored for prolonged periods, be this in or ex situ, this could lead to malformation of the gametophytes (Quintanilla et al, 2002).
As Woodsia ilvensis grows on rocky outcrops, how accessible and viable would a soil spore bank be?



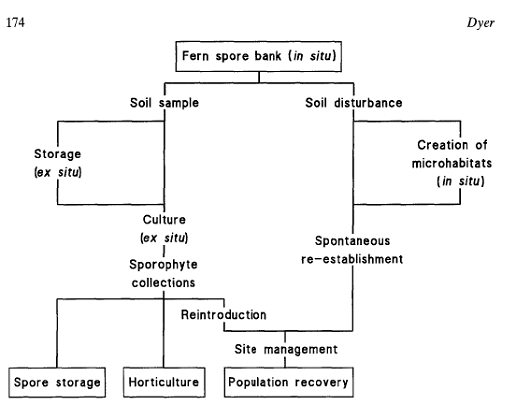
Fig:7 population recovery from soil spore banks
(Source : Dyer ,1994)
Perhaps the only spores to germinate after prolonged storage are spores genetically fittest for storage, this may alter the gene pool in some way?
With climate changing rapidly at present, spores stored for decades may not be fit for their natural habitat at the time of sowing.
Acknowledgements:
Our thanks to Dr. Heather McHaffie, Dr. Maria Chamberlain and Mike Dye for their guidance and support.
References:
Dyer A.F. (1994) Natural soil spore banks- can they be used to retrieve lost ferns? Biodiversity and Conservation 3, 160-175 1994 Chapman & Hall.
Dyer A.F. and Lindsay S. (1992) Soil Spore Banks of Temperate Ferns. American fern journal 82(3): 89-122.
Ellwood M., Jones D., Foster W. (2002)
Canopy Ferns in
Fitter R., Fitter
A. and Farrer A. (1984) Collins guide to
the Grasses, Sedges, Rushes and Ferns of Britain and Northern Europe
William Collins & Sons Co Ltd,
Lusby P. and Wright J. (2001) Scottish Wild Plants: Their History, Ecology and Conservation. Mercat Press
McHaffie, H. (2008). Fern Spore Viability [Presentation].
Quintanilla L.G., Amigo J., Pangua E. and Pajarn S. (2002) Effect of storage method on spore viability in five globally threatened fern species. Annals of Botany 90: 461-467.
Smith, G.C.,
Available online from:
Soeder RW (1985) Fern constituents: including occurrence, chemotaxonomy and
physiological activity. Bot. Rev. 51,442-536.
Thomas, J.A.,
Telfer, M.G.,
Available online from:
UK Biodiversity Group (1998) UK Biodiversity Group Tranche 2 Action Plans - Volume I: Vertebrates and vascular plants, Species Action Plan Oblong Woodsia (Woodsia ilvensis).
Available online from:
Wagner, G.M. (1997) Azolla: A Review of Its Biology and Utilization. Botanical Review, 63 (1).
Waller P., Bernes G, Thamsborg SM, Sukura A., Richter SH, Ingebrigtsen K, Hglund J.(2000) Plants as De-Worming Agents of Livestock in the Nordic Countries: Historical Perspective, Popular Beliefs and Prospects for the Future. Available online from:
IUCN 2007. 2007 IUCN Red List of Threatened Species.
The
www.ukbap.org.uk
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 5088
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2025 . All rights reserved