| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
Structure Determination:
Nuclear Magnetic Resonance Spectroscopy
1a. Gjr kort rede for NMR spektroskopi.
Lreboken side 424-429.
1b. Gjr rede for skjerming av kjerner og om hvordan nrliggende elektronnegative kjerner vil pavirke skjermingen av kjerner.
Lreboken side 426-427
1c. Grei ut om spin-spin splitting i 1H NMR spektre.
Lreboken side 443-447.
Nuclear magnetic resonance spectroscopy provides information about a molecule's:
a. conjugated pi electron system
b. size and formula.
c. carbon-hydrogen framework.
d. functional groups.
Answer: c
Explain why all protons in a molecule do not absorb rf energy at the same frequency.
Answer: All nuclei in molecules are surrounded by electron clouds. When a uniform external magnetic field is applied to a molecule, the circulating electron clouds set up tiny local magnetic fields of their own. These local magnetic fields act in opposition to the applied field, so that the effective field actually felt by a nucleus is a bit smaller than the applied field.
Beffective = Bapplied - Blocal
This effect is termed shielding. Each nucleus is shielded to a slightly different extent, so each unique kind of proton in a molecule resonates at a slightly different frequency and gives rise to a unique NMR signal.
The following questions pertain to the charting of NMR spectra. MATCH a term to each description below. Place the letter of the term in the blank to the left of the description.
a. TMS
b. high-field or upfield side
c. MHz
d. delta ![]()
e. low-field or downfield side
f. chemical shift
g. specific absorption
When looking at an NMR chart the right-hand part of the chart is the .
Answer: b
The exact place on the chart at which a nucleus absorbs is called its .
Answer: f
The calibration standard for ![]() and
and ![]() NMR is:
NMR is:
Answer: a
The NMR charts are calibrated using an arbitrary scale that is divided into units.
Answer: d
For each of the compounds below tell how many
signals you would expect the molecule to have in its normal, broadband
decoupled ![]() NMR spectra.
NMR spectra.
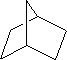
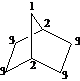
Answer: three
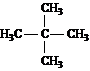
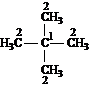
Answer: two
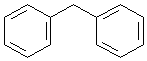

Answer: five
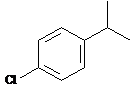
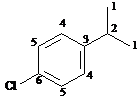
Answer: six
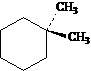
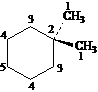
Answer: five
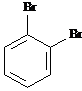
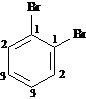
Answer: three
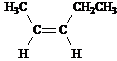
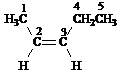
Answer: five
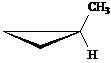
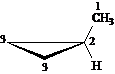
Answer: three
Identify the indicated sets of protons as unrelated, homotopic, enantiotopic, or diastereotopic.
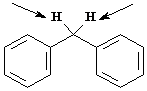
Answer: homotopic
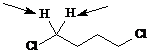
Answer: enantiotopic
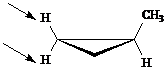
Answer: diastereotopic
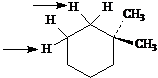
Answer: unrelated
For each compound below tell how many types of nonequivalent protons there are.
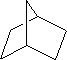
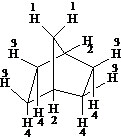
Answer: four
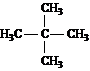
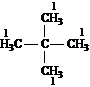
Answer: one

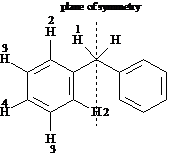
Answer: four

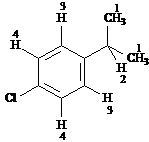
Answer: four
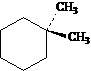
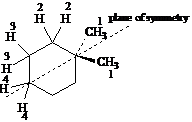
Answer: four
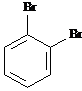
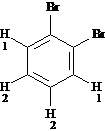
Answer: two
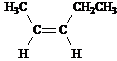
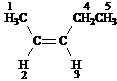
Answer: five

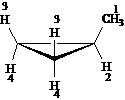
Answer: four
Predict the splitting patterns you would expect for each proton in the molecules below:
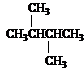
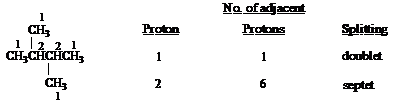
Answer:
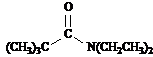
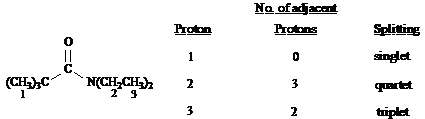
Answer:
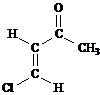
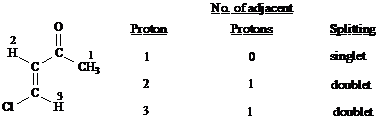
Answer:
The ![]() NMR spectrum of
styrene oxide shows that protons 1, 2, and 3 all have different chemical shift
values. Proton 1 is coupled to both
proton 2
NMR spectrum of
styrene oxide shows that protons 1, 2, and 3 all have different chemical shift
values. Proton 1 is coupled to both
proton 2 ![]() and proton 3
and proton 3 ![]() . Draw a tree diagram
for the proton 2 signal.
. Draw a tree diagram
for the proton 2 signal.
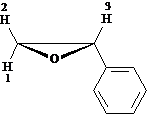
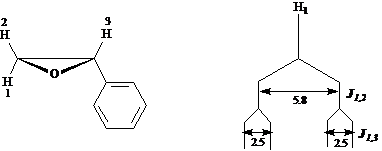
Answer:
Refer to the structure of 3-methyl-2-butanone below to answer the following questions.
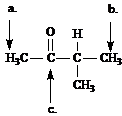
What is the splitting pattern for the hydrogens in 3-methyl-2-butanone labeled a.?
a. septet
b. quartet
c. doublet
d. singlet
Answer: d
What is the splitting pattern for the hydrogens in 3-methyl-2-butanone labeled b.?
a. septet
b. quartet
c. doublet
d. singlet
Answer: c
The carbonyl-carbon resonance of 3-methyl- 2-butanone occurs at 208.7 ppm downfield from TMS. How many hertz downfield from TMS would this carbonyl-carbon absorb is the spectrometer used to measure this absorption were operating at 200 MHz?
|
Answer: |
|
Treatment of tert-butyl alcohol with hydrogen
chloride yields a mixture of tert-butyl
chloride (SN1 product) and 2-methylpropene (E1 product). After chromatographic separation, how would
you use ![]() NMR to help you decide
which was which?
NMR to help you decide
which was which?
![]()
Answer: 2-Methylpropene has two kinds of hydrogens. It will have a vinylic absorption (4.56.5 ) representing two hydrogens and an unsplit signal (1.01.5 ) due to the six equivalent methyl hydrogens.
tert-Butyl chloride has only one kind of hydrogen, which results in one unsplit signal.
Below are three isomeric chlorobutanes and their ![]() NMR spectral
data. MATCH the spectral data to the
correct structures by placing the letter of the spectrum in the blank to the
left of the corresponding structure.
NMR spectral
data. MATCH the spectral data to the
correct structures by placing the letter of the spectrum in the blank to the
left of the corresponding structure.
a ![]() , 55.4, 36.2, 19.3
, 55.4, 36.2, 19.3
b ![]() , 56.2, 35.3
, 56.2, 35.3
c ![]() , 56.0, 36.0, 24.2, 8.2
, 56.0, 36.0, 24.2, 8.2
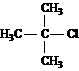
Answer: b
![]()
Answer: c
![]()
Answer: a
Propose
structures for compounds that fit the following ![]() NMR data:
NMR data:
![]()
3H doublet at 2.0 , ![]()
1H quartet at 5.0 , ![]()
5H singlet at 7.3
Answer:
![]()
6H triplet at 0.9 , ![]()
4H sextet at 1.6 , ![]()
4H triplet at 2.4 , ![]()
![]()
Answer:
![]()
2H quintet at 2.4 , ![]()
4H triplet at 3.5 , ![]()
Answer: ![]()
![]()
6H doublet at 1.2 , ![]()
3H singlet at 2.3
1H septet at 2.9 , ![]()
4H singlet at 7.0
Answer:
![]()
12H doublet at 0.8
2H septet at 1.4
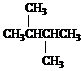
Answer:
To answer the
following questions, consider the data and![]() NMR spectrum below:
NMR spectrum below:
2H 2H 3
H
The mass spectrum of
this compound shows a molecular ion at m/z
= 113, the IR spectrum has characteristic absorptions at 2270 and 1735 cm-1,
and the 13C NMR spectrum has five signals.
Spectrum obtained from: SDBSWeb: https://www.aist.go.jp/RIODB/SDBS/
Based on the mass spectral data and the IR data, what functional groups are present in this compound?
Answer: A molecular ion of mass 113 indicates an odd number of nitrogen atoms in the compound. Coupled with the IR absorption at 2270 cm-1, a nitrile functional group is indicated. The IR absorption at 1735 cm-1 indicates a carbonyl group, possibly an ester.
How many types of nonequivalent protons are there in this molecule?
Answer: three
Describe the signal at 3.5 d in terms of its integration, splitting pattern and chemical shift.
Answer: The integration of the signal at 3.5 d indicates that it is due to two equivalent hydrogens, probably a -CH2- group. Since the signal is a singlet (not split) there are no nonequivalent hydrogens attached to the atoms adjacent to the carbon to which these two hydrogens are bonded. The chemical shift to 3.5 d indicates that this -CH2- group has at least one electronegative atom or group bonded to it, possibly a carbonyl group
.
Describe the signals at 4.35 d and 1.3 d in terms of their integration, splitting and chemical shift.
Answer: The signal at 4.35 d is owing to two equivalent hydrogens split by three adjacent hydrogens or a -CH2- next to a -CH3. It is shifted by attachment to an electronegative atom like oxygen. The signal at 1.3 d is three equivalent hydrogens split by two adjacent hydrogens of a -CH3 next to a -CH2-. It is shifted slightly downfield by the presence of an electronegative atom bonded to the adjacent -CH2-
What is the significance of the 13C NMR data?
Answer: The 13C NMR has five signals, which means that there five differenct kinds of carbon in this compound.
Propose a structure for this compound.
Answer: By compiling all the information deduced from the data provided, we note that this compound is a) a nitrile and an ester [two carbons accounted for], and b) has two CH2 groups and one CH3 group [three more carbons accounted for]. One of the CH2 groups is attached to the oxygen of the ester and one is attached to the carbonyl of the ester. The singlet CH2 is also bonded to the nitrile. Based on the chemical shifts, the singlet CH2 must be bonded to the carbonyl and the quartet CH2 must be bonded to the oxygen of the ester. So the compound is ethyl cyanoacetate:
![]()
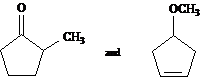
How would you use ![]() and
and ![]() NMR to help you
distinguish between these two isomeric structures?
NMR to help you
distinguish between these two isomeric structures?
|
|
Answer: |
# peaks |
Distinguishing Absorptions |
||
|
|
methyl doublet at 1.5 (overlaps other methylene signals), no vinyl protons one carbonyl carbon, no vinyl carbons |
||||
|
| |||||
|
|
split vinylic peak; rel. area 2 methyl singlet between 3.54.0 one vinylic carbon, no carbonyl carbon |
||||
Answer the
questions for each of the compounds whose ![]() NMR spectra are shown
below.
NMR spectra are shown
below.
3H 3H
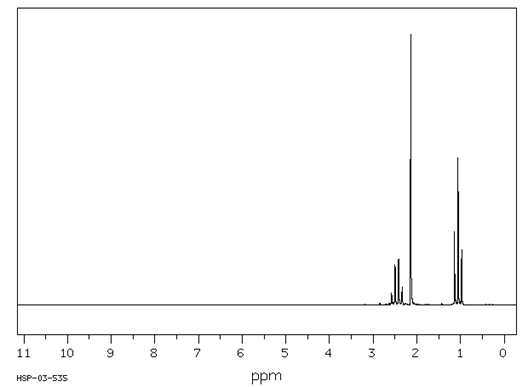
![]()
2H
Spectrum obtained from: SDBSWeb: https://www.aist.go.jp/RIODB/SDBS/
Describe each signal in terms of its integration, splitting and chemical shift.
Answer: The signals at 1.05 and 2.45 d are characteristic, in their integration and slitting (a 2H quartet and a 3H triplet), for an ethyl group. The shift of the CH2 to 2.45 indicates that the ethyl group is attached to a carbonyl group. The signal at 2.1 d, a 3H singlet, is characteristic for a CH3 attached to a carbonyl group.
Propose a structure for this compound.
Answer: 2-butanone, ![]()
1H 3H
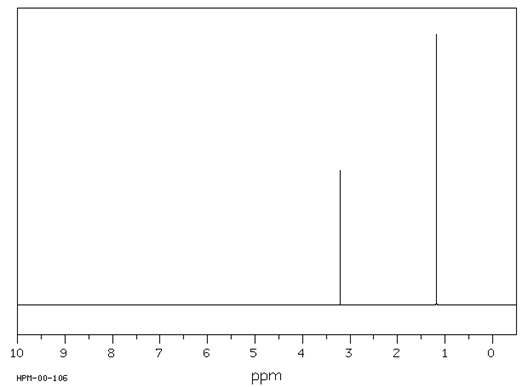
C5H12O
Spectrum obtained from: SDBSWeb: https://www.aist.go.jp/RIODB/SDBS/
Calculate the degree of unsaturation for this compound.
Answer: The base formula for this compound is C5H12, which is corresponds to a saturated compound, so there is no unsaturation in this compound.
Describe each signal in the 1H NMR in terms of its integration, splitting and chemical shift.
Answer: In looking at the spectrum, it is important to note that the relative number of hydrogens for each signal adds up to four. The formula of the compound indicates that there are a total of 12 hydrogens. Therefore, the integration of 3:1 corresponds to 9H:3H. The signal at 1.2 d is a 9H singlet, which is characteristic for a tert-butyl group. The slight downfield shifts indicates that this group is attached to an electronegative atom, in this case, oxygen. The signal at 3.2 d is a 3H singlet which is characteristic of a methyl group bonded to an oxygen.
Propose a structure for this compound.
Answer: tert-butyl methyl ether, (CH3)3COCH3
![]()
3H 4H
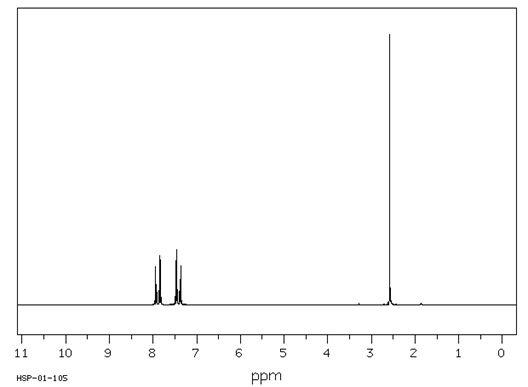
Spectrum obtained from: SDBSWeb: https://www.aist.go.jp/RIODB/SDBS/
Calculate the degree of unsaturation in this compound.
Answer: The base formula for this compound is C8H8; the saturated formula is C8H18; the degree of unsaturation is five [(18 - 8)
Describe the signals that occur between 7 and 8 d in terms of integration, splitting and chemical shift.
Answer: The chemical shift of the signals between 7 and 8 d indicates that these hydrogens are aromatic. The integration of four hydrogens and the splitting pattern of two doublets is characteristic of a para disubstituted aromatic compound.
Describe the signal at 2.6 d in terms of integration, splitting and chemical shift.
Answer: This signal is a 3H singlet shifted by attachment to a carbonyl group; a methyl ketone, probably.
Propose a structure for this compound.
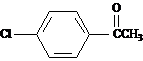
Answer: 4-chloroacetophenone
Which structure of molecular formula C4H8Cl2 fits both the 1H NMR and 13C NMR spectra shown below?
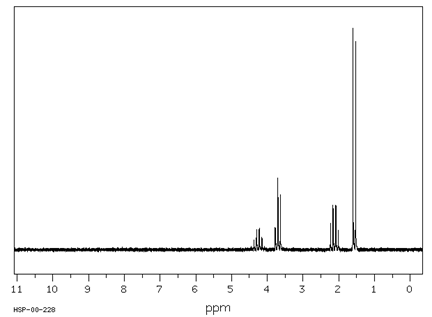
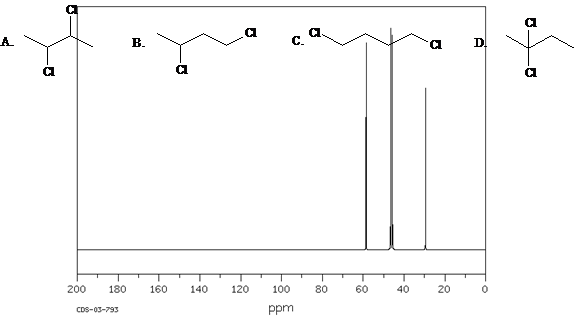
Spectra obtained from: SDBSWeb: https://www.aist.go.jp/RIODB/SDBS/
Answer: B.
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 7206
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2025 . All rights reserved