| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
Abdominal Perfusion Pressure
|
E |
levated intra-abdominal pressure (LAP) is commonly encountered in the critically ill and is associated with significant morbidity and mortality. The 'critical IAP' that causes end-organ dysfunction varies from patient to patient as a result of each individual's physiology and preexisting comorbidities. As a result, a single threshold value of IAP cannot be globally applied to the decision making of all critically ill patients. Calculation of 'abdominal perfusion pressure' (APP), defined as mean arte rial pressure (MAP) minus IAP, assesses not only the severity of IAP present, but also the adequacy of the patient's abdominal blood flow. APP is superior to both LAP and global resuscitation endpoints such as arterial pH, base deficit, and arterial lactate in its ability to both predict patient outcome and serve as a useful parameter for guiding the resuscitation and management of the patient with IAH or ACS.
Introduction
Although initially recognized almost 150 years ago, the pathophysiologic implications of elevated intra-abdominal pressure (IAP) have essentially been rediscovered only within the past decade. l'15 Elevated LAP or 'intra-abdominal hypertension' (IAH) is now commonly identified in the critically ill and acknowledged as a cause of significant morbidity and mortality.7'16'ls IAH has been recognized as a continuum of pathophysiologic changes beginning with regional blood flow disturbances and ultimately culminating in the LAP-induced end-organ failure now known as the 'abdominal compartment syndrome' (ACS). A recent multi-center epidemiologic study found IAH (defined as IAP >_ 12 mm Hg) to be present in 51% of critically ill medical and surgical intensive care unit (ICU) patients and ACS (defined as IAP >_ 20 mm Hg with one or more organ failures) to be present in 8%.16 The prevalence of elevated LAP in patients developing organ failure suggests that IAH may well play a major role in the development of multiple system organ failure (MSOF), a major cause of ICU mortality.
Further complicating the diagnosis and management of these critically ill patients is the fact that IAH is difficult to detect by physical examination alone.' 9 Elevated IAP can easily go undetected resulting in significant end-organ dysfunction and failure. Assessment of LAP, most commonly using the surrogate measurement of intravesicular or 'bladder' pressure, has been identified as being essential to the accurate diagnosis and treatment of patients with IAH or ACS.Z'Z3 Given the prevalence of IAH in the ICU, the difficulty of diagnosis, and the significant associated morbidity and mortality, the importance of measuring IAP, identifying the presence of end-organ malperfusion, and restoring both systemic and regional organ perfusion in patients with IAH or ACS cannot be overemphasized.
the detrimental effects of elevated IAP has evolved, so has our understanding of what constitutes IAH. Whereas early studies suggested that an IAP of 30 to 40 mm Hg was acceptable, we now recognize that even small elevations in UP to 10 to 15 mm Hg can have a tremendous impact on end-organ perfusion and patient outcome. As a result, the definition of IAH has required continual adjustment over the years and the 'critical IAP' that mandates intervention has been revised downward .5,7,18,22 The patient populations at risk for developing LAB and ACS have also been expanded. Originally considered a disease of the traumatically injured, IAH and ACS have now been recognized to occur in virtually all patient populations. The acuity of onset and presence of preexisting comorbid conditions have been identified to exert a significant impact upon the presentation and management of elevated IAP Age, obesity, prior pregnancy, mechanism of injury, pulmonary, cardiac, or renal dysfunction or failure, need for mechanical ventilation, and development of systemic inflammatory response syndrome (SIRS) have all been recognized to modulate the development and appropriate treatment of these critically ill patients.
During the early evolution of our understanding of IAH and ACS, attempts were made to identify a single threshold value of IAP that could be simply and globally applied to the decision making of all critically ill patients with IAH. The 'critical IAP' that mandates intervention may well be considered the 'holy grail' of IAH and ACS research. Such a quest, however, oversimplifies what is actually a highly complex and variable physiologic process. While IAP is a major determinant of patient outcome during critical illness, the IAP that defines IAH and ACS clearly varies from patient to patient and even within the same patient as their disease process evolves. Within the clinically acceptable ranges, IAP is a specific, but nonsensitive predictor of illness and resuscitation adequacy. Improving the diagnostic sensitivity of IAP would require allowing patients to maintain higher IAP values. Such levels of IAH, however, are well-known to cause end-organ malperfusion and such a practice would not be clinically acceptable given our current understanding of the morbidity and mortality of IAH. Thus, although a useful screening tool, IAP lacks sufficient sensitivity to be useful as a resuscitation endpoint. Given the marked variation in IAP values that may be witnessed in the critically ill, it is unlikely that a single threshold value of IAP will ever be universally applicable to all critically ill patients.
An alternative approach to improving the sensitivity of LAP is to incorporate it in the assessment of abdominal perfusion as a resuscitation endpoint. Analogous to the widely accepted and utilized concept of cerebral perfusion pressure (CPP), calculated as mean arterial pressure (MAP) minus intracranial pressure (ICP), the abdominal perfusion pressure (APP), calculated as NW minus IAP, has been proposed as a more accurate marker of critical illness and endpoint for resuscitation. 11,14 This chapter evaluates the scientific support for and potential clinical application of APP in the management of patients with IAH and ACS.
APP = MAP - UP Physiology
Elevated IAP causes significant impairment of cardiac, pulmonary, renal, gastrointestinal, and hepatic function with each organ demonstrating its own unique vulnerability. The IAP that may induce malperfusion in one organ system may have little effect on another. This differential response, coupled with the augmented susceptibility to IAP seen with hypovolemia and comorbid disease, complicates the management of these critically ill patients and use of UP as a resuscitative endpoint. It also emphasizes the importance of assessing perfusion pressure as opposed to compartment pressure alone. The detrimental effect of IAP on individual organ systems is discussed in detail in other chapters; the following summarizes the salient points as they pertain to the validity of IAP and APP as resuscitative endpoints.
Cardiovascular
As originally described over 80 years ago by Emerson, rising IAP increases intrathoracic pressure through cephalad deviation of the diaphragm. 25 Increased intrathoracic pressure significantly reduces venous return and cardiac output and compresses both the aorta and pulmonary parenchyma raising systemic vascular resistance. Such reductions have been demonstrated to occur at an IAP of only 10 mm Hg. Hypovolemic patients, those with marginal cardiac contractility, and those requiring positive pressure ventilation appear to sustain reductions in cardiac output at lower levels of IAP than do normovolemic patients.
Pulmonary
Increases in intrathoracic pressure, through cephalad elevation of the diaphragm, also result in extrinsic compression of the pulmonary parenchyma with development of alveolar atelectasis, decreased diffusion of oxygen and carbon dioxide across the pulmonary capillary membrane, and increased intrapulmonary shunt fraction and alveolar dead space. This dysfunction begins at an IAP of 15 mm Hg and is accentuated by the presence of hypovolemia.3o In combination, these effects lead to the arterial hypoxemia and hypercarbia that characterize AC$.7'2G,30
Renal
Elevated IAP significantly decreases renal artery blood flow and compresses the renal vein leading to renal dysfunction and failure. 31,32 Oliguria develops at an UP of 15 mm Hg and anuria at 30 mm H~ in the presence of normovolemia and at lower levels of IAP in the patient with hypovolemia. Renal perfusion pressure (RPP) and renal filtration gradient (FG) have been proposed as key factors in the development of IAP-induced renal failure. The FG is the mechanical force across the glomerulus and equals the difference between the glomerular filtration pressure (GFP) and the proximal tubular pressure (PTP).
FG = GFP - PTP
In the presence of IAH, PTP may be assumed to equal IAP and GFP can be estimated as MAP - UP The FG can then be calculated by the formula:
FG = MAP - 2 x IAP
Thus, changes in IAP have a greater impact upon renal function and urine production than will changes in MAP It should not be surprising, therefore, that decreased renal function, as evidenced by development of oliguria, is one of the first visible signs of IAH. Gastrointestinal
The gut appears to be particularly sensitive to IAH with virtually all intra-abdominal and retroperitoneal organs demonstrating decreased blood flow in the presence of elevated IAP34 Reductions in mesenteric blood flow may appear with an IAP of only 10 mm H g.35 Celiac artery blood flow is reduced by up to 43% and superior mesenteric artery blood flow by as much as 69% in the presence of an IAP of 40 mm He. The negative effects of IAP on mesenteric perfusion are augmented by the presence of hypovolemia or hemorrhage.' 1'2e'35 Bowel ischemia and inadequate perfusion initiates a vicious cycle of worsening perfusion, increased capillary leak, decreased intramucosal pH, and systemic metabolic acidosis. An IAP of 20 mm Hg diminishes intestinal mucosal perfusion and has been speculated as a possible mechanism for subsequent development of bacterial translocation, sepsis, and MSOE t t,14,28,3G-38 Bacterial translocation to mesenteric lymph nodes has been demonstrated to occur in the presence of hemorrhage with an IAP of only 10 mm Hg.
Table 1. The cranian and abdominal compartments
Cranium Abdomen
Organ(s) Brain Liver, spleen, kidneys, stomach, intestines
Fluid(s) Cerebrospinal fluid Ascites, air, feces
Enclosure Skull Abdominal cage
Lesions Tumor, hematoma Blood, edema, ascites, air, tumor
Pressure ICP IAP
Perfusion CPP = MAP - ICP APP = MAP - IAP
ICP: intracranial pressure; IAP: intra-abdominal pressure, CPP: cerebral perfusion pressure MAP: mean arterial pressure, APP: abdominal perfusion pressure
Hepatic
Hepatic artery blood flow is directly affected by UP-induced decreases in cardiac output while hepatic vein and portal vein blood flow are reduced by extrinsic compression.z8 These changes have been documented with UP elevations of only 10 mm Hg and in the presence of both normal cardiac output and mean arterial blood pressure. 28
Theory
The perfusion pressure of any anatomic compartment is dependent upon three factors 1, the arterial inflow pressure
2. the venous outflow pressure
3. the compliance or ability of the compartment to expand in response to increases in volume Perhaps the most clinically accepted example of perfusion pressure is that of the traumatized brain. As the brain is enclosed within a bony 'box' and generally cannot expand past the confines of the skull, cranial compliance becomes a minor issue and CPP may be calculated as arterial inflow (MAP) minus venous outflow (ICP). MAP is determined by intravascular volume, cardiac contractility, and systemic vascular resistance while ICP is dependent upon the respective volumes of the brain, cerebrospinal fluid, intracranial blood, and any space-occupying lesion such as hematoma or tumor. By the Monro-Kellie Doctrine, any increase in the volume of one or more of these four constituents of the cranium will result in an increase in ICP Optimization of CPP is a matter of either increasing MAP (through use of appropriate resuscitation fluids or vasopressors) or decreasing ICP (through administration of diuretics, drainage of cerebrospinal fluid, or evacuation of space-occupying lesions). Interestingly, recent studies have resurrected the concept of hemicraniectomy (removal of a large portion of the skull) to increase cranial compliance much as abdominal decompression increases compliance of the abdominal cavity. 39
Abdominal perfusion may be considered analogous to that of the brain (Table 1). The abdomen contains a variety of solid organs (liver, kidneys, spleen, etc.) and fluids (blood, urine, enteric contents, etc.) of limited compliance, but also air-filled structures (stomach, small intestine, colon) of marked distensibiliry as well as potential spaces (peritoneum, retroperitoneum) that may expand significantly in response to injury or illness. The abdomen may also contain pathologic space-occupying lesions such as blood, air, ascites> or tumor. As with the Monro-Kellie Doctrine and the brain, an increase in the volume of the abdominal cavity contents will result in an increase in IAI'. Although not enclosed in a rigid shell as is the brain, the abdomen is far from being freely compliant and expandable. Portions of the abdomen, such as the spine, pelvis, and costal arch, are fairly rigid while others, such as the diaphragm and especially the abdominal wall, are compliant only up to a point determined by a variety of factors. Age, obesity, abdominal wall musculature, prior pregnancy, and previous abdominal surgery may
each alter abdominal wall compliance. Pain and third-space edema also impair compliance and augment the effects of elevated LAP All of these factors may significantly impact upon the patient's ability to tolerate IAH as well as the adequacy of abdominal perfusion.
Clinical Studies
Cheatham et al first proposed the concept of APP as a predictor of survival in patients with IAH or ACS in 2000.18 A retrospective study was performed evaluating all patients admitted to a surgical / trauma ICU with evidence of IAH (defined as IAP >_ 15 mm Hg). IAP monitoring was instituted whenever one or more signs of IAH-induced organ dysfunction were present including abdominal distention, oliguria refractory to volume administration, hypercarbia, hypoxemia refractory to increasing inspired oxygen fractions and positive end-expiratory pressure, elevated peak inspiratory pressures, or refractory hypotension. IAP monitoring was also instituted in the absence of the above conditions if there was sufficient physician concern for the presence of elevated LAP. Intravesicular pressure was measured, using the technique previously described by Cheatham and Safcsak, every 4 hours until either IAP normalized or measurements were stable and the patient was no longer felt to be at risk for IAH-induced organ dysfunction.' Commonly advocated resuscitation endpoints including arterial pH, base deficit, arterial lactate, and hourly urinary output were recorded. Open abdominal decompression and temporary abdominal closure were performed for symptomatic IAH or development of ACS (defined as IAP >_ 25 mm Hg in the presence of one or more signs of IAH-induced organ dysfunction). Multivariate logistic regression analysis was performed to identify those physiologic variables and resuscitation endpoints significantly associated with patient survival.
During the 25-month study period, 144 patients (68% trauma, 14% general surgery, 14% vascular surgery, 2% colorectal surgery, and 2% obstetrics / gynecology) underwent 2298 LAP measurements during resuscitation for IAH or ACS. The mean IAP for all patients was 22 8 mm Hg (range 2-94) despite relatively liberal application of abdominal decompression. On average, patients underwent IAP monitoring for 3 2 days (range 1-11 days) during which time 16 14 measurements (range 1-61) were performed. Overall mortality was 53%. Significantly fewer patients developed ACS in the second half of the study (64% vs. 43%; p = 0.01) and mortality was significantly decreased (44% vs. 28%; p = 0.049) as a result of physician education and increased acceptance of abdominal decompression.
Multiple logistic regression analysis demonstrated that LAY, MAP, APP, arterial lactate, arterial pH, base deficit, and hourly urinary output were all significantly associated with patient survival from IAH with hourly urinary output and APP being most predictive (Table 2). Further analysis utilizing the worst values measured during the patient's resuscitation identified that lowest APP was significantly superior to the other resuscitation endpoints in its ability to predict patient survival from IAH (Table 3).
Receiver operator characteristic (ROC) curves were generated for LAY and APP in order to identify the threshold values of each endpoint that were most predictive of patient outcome. ROC curves graph the sensitivity of a diagnostic test (true positive proportion) versus 1 minus specificity (false positive proportion) and provide an improved measure of the overall discriminatory power of a test as they assess all possible threshold values. A test that always predicts survival has an area under the ROC curve of 1.0 and a test that predicts survival no more often than would be done by chance has an area under the ROC curve of 0.5. The point on the ROC curve closest to the upper left corner is generally considered to optimize the sensitivity and specificity of the test. In this study, the area under the ROC curve was 0.726 for APP and 0.748 for IAP (Fig. 1; IAP has been plotted against mortality instead of survival as in the original study). 18 Although the areas under the ROC curves for APP and IAP are not statistically different, the curves demonstrate that the sensitivity and specificity of APP are both superior to that of IAP for the clinically useful decision thresholds (Table 4). Maintenance of an APP of at least 50 mm Hg appears to maximize both the sensitivity (76%) and specificity (57%) of APP as a predictor of patient survival. The commonly utilized MAP resuscitation endpoint of 70 mm Hg achieved a sensitivity of only 57% and specificity of 61%. While an IAP threshold of 30
Table 2. Resuscitative endpoints in IAH /ACS stratified by survival'
Survivors / Significance of Multiple Non-Survivors / Logistic Regression
Hourly urine output (m l/h) 113 112 79 111 < 0.0001
APP (mm Hg) 69 17 61 18 0.0001
Arterial I actate (mmol/L) 2.9 1.5 4.5 2.5 0.0002
MAP (mm Hg) 88 15 85 15 0.0004
Arterial pH 7.34 0.08 7.27 0.10 0.03
Base deficit 3.6 4.8 7.5 5.6 0.04
IAP (mm Hg) 20 6 24 8 0.05
MAP: mean arterial pressure; IAP: intra-abdominal pressure; APP: abdominal perfusion pressure
mm Hg achieved a sensitivity of 70% and specificity of 72%, this endpoint exceeds what is now recognized as being clinically acceptable and its application would place the patient at risk for significant end-organ malperfusion. Within the currently advocated ranges of 10 to 25 mm Hg, Up was specific, but not sensitive for predicting patient outcome. APP appears to be a clinically superior resuscitation endpoint and predictor of patient survival during treatment of IAH and ACS as it addresses not only the severity of IAH, but also the adequacy of end-organ perfusion.
To evaluate the clinical validity of various resuscitation endpoints, including APP, in patients with IAH, Malbrain et al prospectively evaluated 8 patients treated by their surgeon with an abdominal binder to reduce postoperative dehiscence and incisional hernia. 24 IAP was noted to increase significantly upon application of the abdominal binder with significant decreases in cardiac output and increases in central venous and pulmonary artery occlusion pressure appearing within 30 to 45 minutes. With elevations in UP, visceral perfusion worsened as evidenced by decreased APP, FG, arterial pH, intramucosal pH (pHi), and arterial-gastric mucosal carbon dioxide difference (C02-gap). The addition of positive end-expiratory pressure (PEEP) of 15 cm H20 in combination with elevated IAP had even greater deleterious effects on APP and visceral perfusion. These effects were reversible with release of the abdominal binder (mimicking abdominal decompression) and a decrease in PEEP This study confirmed the significant deleterious impact of both elevated IAP and intrathoracic pressure on APP and
Table 3. Outcome variables in IAH /ACS stratified by Survival'
Survivors /Significance of Multiple Non-Survivors / Logistic Regression
Lowest APP (mm Hg) 52 17 39 18 0.002
Lowest MAP (mm Hg) 74 14 69 14 0.05
Highest IAP (mm Hg) 29 12 38 14 0.21
Highest arterial lactate (mmol/L) 5.4 2.2 8.0 3.7 0.38
Highest base deficit 9.0 7.0 13.1 6.9 0.44
Lowest arterial pH 7.24 0.10 7.14 0.13 0.66
Lowest hourly 47 48 44 68 0.85
urinary output (mL/h)
MAP: mean arterial pressure; IAP: intra-abdominal pressure; APP: abdominal perfusion pressure
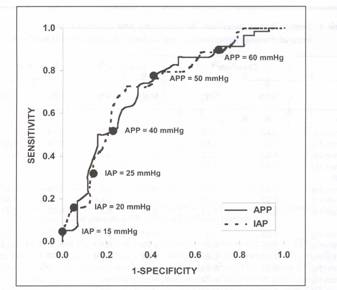
Figure 1. Receiver operator characteristic (ROC) curves for IAP and APP with clinically useful decision points. IAP: intra-abdominal pressure; APP: abdominal perfusion pressure
visceral perfusion. Abdominal decompression increased APP and decreased IAP restoring abdominal perfusion. Regional markers of perfusion adequacy such as APP, FG> pHi, and C02-gap were noted to change more rapidly than global indices such as MAP, arterial pH, base deficit, and calculated bicarbonate (HC03 ) suggesting that the regional markers are superior resuscitation endpoints.
Malbrain et al subsequently prospectively studied the development of IAH in 405 patients admitted to a mixed ICU over a 12-month period.Z4 IAP was routinely assessed in all patients and the maximal LAP and lowest APP values measured within the first 72 hours were recorded. IAH (defined as IAP > 12 mm Hg) was associated with a significantly higher ICU mortality (65% vs. 8%; p<0.0001) and hospital mortality (69% vs. 18%; p<0.0001). APP was significantly lower among nonsurvivors (61 23 vs. 76 23 mm Hg; p<0.0001) as was MAP (72 22 vs. 83 22 mm Hg; p<0.0001) while LAP was significantly higher (11 5 vs. 74 mm Hg; p<0.0001). An APP of 60 mm Hg was identified as having a sensitivity of 55% and specificity of 76% for predicting survival while a MAP of 70 mm Hg had a sensitivity of 58% and specificity of 64% (Table 4). An IAP of 9 mm Hg had the best sensitivity (65%) and specificity (72%) for predicting patient outcome, however, this IAP threshold may be unrealistic for a more critically ill patient population. As only 18% of the patients in this study had evidence of IAH and only 2% had ACS (defined as IAP > 20 mm Hg), the findings of this study suggest that APP may have application as a resuscitative endpoint in not only the patient with IAH or ACS, but in the broader ICU patient population as well.
The Critically Ill and Abdominal Hypertension (CIAH) study group subsequently performed a prospective international multi-center trial in which 257 patients were screened for IAH (defined as IAP >_ 12 mm Hg).24 Patient demographics included 47% medical, 28% elective surgery, 17% emergency surgery, and 9% trauma. Overall hospital mortality was 35% (48% medical, 11% elective surgery, 44% emergency surgery, 18% trauma). Conditions
Table 4. Sensitivity and specificity for predicting patient survival from intra-abdominal hypertension and abdominal compartment syndrome
Cheatham1e Malbrain24 CIAH24
SENS SPEC SENS SPEC SENS SPEC
APP
40 mm Hg 0.53 0.78 0 .14 0.98 0.14 0.95
50 mm Hg 0.76 0.55 0.39 0.91 0.53 0.85
60 mm Hg 0.92 0.25 0.55 0.76 0.79 0.62
70 mm Hg 0.97 0.18
MAP
60 mm Hg 0.23 0.87 0.34 0.87 0.40 0.87
70 mm Hg 0.57 0.60 0.58 0.64 0.69 0.61
80 mm Hg 0.83 0.21 0.74 0.42 0.85 0.41
IAP
12 mm Hg 0.05 1.0 0.44 0.93 0.75 0.59
15 mm Hg 0.05 1.0 0.23 0.97 0.47 0.75
20 mm Hg 0.16 0.85 0.06 0.99 0.17 0.92
25 mm Hg 0.32 0.86
30 mm Hg 0.70 0.72
35 mm Hg 0.80 0.47
40 mm Hg 0.89 0.32
MAP: mean arterial pressure; IAP: intra-abdominal pressure; APP: abdominal perfusion pressure
associated with the development of IAH included acidosis, hypothermia, polytransfusion, volume resuscitation, coagulopathy, sepsis, abdominal surgery, ileus, and hepatic dysfunction. IAP was significantly correlated with the development of both organ failure and mortality. APP was significantly lower among nonsurvivors (54 16 vs. 69 23 mm Hg; p<0.0001) as was MAP (68 15 vs. 81 23 mm Hg; p<0.0001) while IAP was significantly higher (15 6 vs. 12 5 mm Hg; p<0.0001). Within the subgroup of 145 patients with IAH, APP continued to be significantly lower among nonsurvivors (52 14 vs. 65 23 mm Hg; p<0.001) while IAP was no longer different (17 5 vs. 17 4 mm Hg) confirming the superiority of APP as a predictor of clinical outcome. The area under the ROC curve was 0.732 for APP and 0.678 for IAP An APP of 60 mm Hg was associated with the best sensitivity (79%) and specificity (62%) while a MAP of 70 mm Hg had a sensitivity of 69% and specificity of 61 %. An IAP threshold of 12 mm Hg had a sensitivity of 75% and specificity of 59%. Based upon these findings, the CIAH study group recommended maintaining an APP above 60 mm Hg and IAP below 12 mm Hg to optimize patient outcome.
The CIRFAH (Critically Ill Renal Failure and Abdominal Hypertension) study prospectively evaluated IAP and APP as predictors of outcome in 60 patients with acute renal failure (ARF) (defined as serum creatinine > 2 mg/dL). Over a 12-month period, patients admitted with or who developed ARF during their ICU stay were screened for IAH (defined as IAP > 12 mm Hg) using intravesicular pressure measurements. IAP was recorded twice daily together with the highest and lowest APP, fluid balance, and Sequential Organ Failure Assessment (SOFA) score. There were 78% medical and 22% surgical patients. The renal Stuivenberg Hospital Acute Renal Failure (SHARF-II) score was 67 22 on admission and 76 24 after 48 hours. The SOFA score on day 1 was 9.4 3.5 with 1.7 1.1 organ failures. The IAP on day 1 was 12 5 mm Hg while APP was 55 18 mm Hg. Maximal IAP after 48 hours (IAPmax) was 14
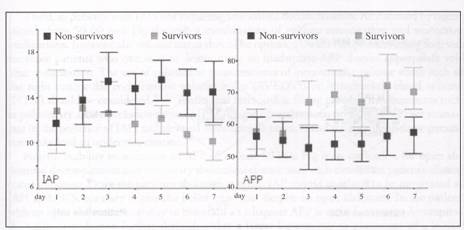
Figure 2. Evolution of IAP and APP in patients with acute renal failure stratified in survivors and non survivors. IAP: intra-abdominal pressure; APP: abdominal perfusion pressure.
6 mm Hg. IAH > 12 mm Hg within 48 hours of study inclusion was present in 63% patients. Outcome data on 46 patients showed a 28-day mortality of 63%. Outcome did not differ by presence of IAH although nonsurvivors had a significantly higher UP and lower APP by day 3 (Fig. 2). There was also a trend towards a more positive daily fluid balance and cumulative net fluid balance in nonsurvivors. Thus, the incidence of IAH is high in patients with ARF and is associated with significant mortality that is underestimated by classic severity scores. The SHARF-II score better predicts outcome in these patients. The persistence of IAH and a low APP by day 3 was able to discriminate between survivors and nonsurvivors. Close monitoring of LAP and APP therefore seems warranted in patients with ARE
Clinical Application
Early goal-directed therapy to restore end-organ perfusion and oxygen delivery at the cellular level is essential to reducing patient morbidity and mortality. As critical IAP varies from patient to patient, IAP alone cannot serve as a therapeutic goal in this patient population. APP, calculated as MAP minus IAP, assesses not only the severity of a patient's UP, but also evaluates the adequacy of their abdominal perfusion. APP provides an easily calculated measure that has been demonstrated to exceed the clinical prediction of IAP alone in early clinical trials. APP improves upon the sensitivity of UP while maintaining diagnostic specificity and appears to correlate well with visceral perfusion. Only time and further experience will determine whether APP, or another as yet undetermined resuscitation endpoint, proves its usefulness in large scale clinical trials.
Merging the results of the above studies with the available clinical literature on the pathophysiologic implications of IAH and management of ACS, an appropriate management algorithm for the patient with elevated IAP can be proposed (Fig. 3). First, serial UP measurements should be performed liberally in the critically ill due to the high incidence of IAH in this patient population and its significant associated morbidity and mortality. Due to the inadequacy of physical examination in detecting elevated IAP, serial UP measurements are currently the only method available by which to accurately diagnose IAH and direct appropriate treatment. Intravesicular pressure monitoring can be performed rapidly and inexpensively without specialized monitoring equipment using materials available in any ICU.21
Second, immediate abdominal decompression should be performed in any patient who demonstrates significant elevations in UP or evidence of ACS. In surgical patients, this is best achieved by either creating or reopening their laparotomy incision and applying a temporary

Figure 3. IAH / ACS resuscitation algorithm. IAH: intra-abdominal hypertension; ACS: abdominal compartment syndrome; IAP: intra-abdominal pressure; APP: abdominal perfusion pressure.
abdominal closure. This can be performed either in the operating room or at the patient's bedside in the ICU based upon their current hemodynamic stability.7'18 Such a procedure should not be feared or delayed as rapid decompression following the diagnosis of ACS dramatically improves patient organ function and survival. 18 A recent long-term outcome study demonstrated no significant residual physical or mental health deficits and a return to normal functionality within 12 months among patients requiring emergent abdominal decompression.' In medical patients whose IAH is secondary to accumulation of ascites or resuscitation fluid, paracentesis should be considered as a viable alternative to open abdominal decompression. Leaving the paracentesis catheter in place until the patient's condition stabilizes allows ongoing drainage of peritoneal fluid, continued reduction in LA-P, and a reduced incidence of tertiary or recurrent ACS. Patients whose IAI-I is secondary to retroperitoneal hemorrhage, visceral edema, or ileus will be best served by open abdominal decompression as paracentesis will not be effective in reducing the severity of IAH or restoring organ perfusion.
Third, in patients with IAH not requiring immediate decompression, APP should be maintained above 50-60 mm Hg through a combination of volume resuscitation and vasoactive medications. Intravascular volume status should be optimized, with vasopressors being reserved for those patients who continue to demonstrate an inadequate APP despite appropriate volume resuscitation. The use of volumetric measurements of intravascular volume status such as the right ventricular end-diastolic volume index (RVEDVI) or intrathoracic blood volume (ITBU) should be considered.4o'41 Traditional intracardiac filling pressure measurements such as pulmonary artery occlusion pressure (PAOP) and central venous pressure (CVP) are inaccurate in the presence of IAH and elevated intrathoracic pressure and reliance upon these parameters may lead to underresuscitation.4o
Fourth, inability to maintain an APP of at least 50 mm Hg is an indication for open abdominal decompression and temporary abdominal closure until such time as the patient's clinical status improves. Once the patient's abdomen is open, IAP should continue to be monitored as IAH and ACS, contrary to popular belief, can recur despite an open abdomen. In the patient with an open abdomen, inability to maintain an adequate APP is an indication to decompress the patient's abdomen further through either a larger laparotomy or placement of a looser, more compliant temporary abdominal closure.
Fifth, attempts to close the patient's abdomen following decompression should be directed by the patient's IAP and APP APP can be utilized to guide not only the difficult question of when to perform a decompressive laparotomy, but also the frequently more complicated decision of when and how to perform abdominal closure. Persistent elevations in IAP with marginal APP calculations should lead to a surgical decision for split-thickness skin grafting of the exposed viscera as opposed to attempts to close the patient's abdominal wall under tension. The latter will undoubtedly result in recurrent IAH, decreased visceral perfusion, and increased risk of incisional dehiscence.
In summary, the simple calculation of APP is superior to UP alone as a resuscitative endpoint in patients with IAH or ACS as it assesses both the severity of IAH and the adequacy of end-organ perfusion. APP is also superior to global markers of resuscitation adequacy such as arterial pH, base deficit, and arterial lactate in its ability to quickly identify inadequate visceral perfusion. An APP of 50 mm Hg or greater should be considered a resuscitation endpoint in the patient with elevated IAP
Commentary Rao R Ivatury
Cheatham and Malbrain, my distinguished co-editors and champions of the cause of intra-abdominal hypertension and the abdominal compartment syndrome, describe in this chapter the concept of abdominal perfusion pressure, which in the future may revolutionize our interpretation of the intra-abdominal pressure. The authors provide strong evidence why the effective 'transmural pressure' (perfusion pressure, compartment perfusion pressure, transabdominal pressure, trans-thoracic pressure and such), modeled along the lines of compartment pressure of the extremities, will be important to be considered.
The authors provide persuasive arguments why APP is superior to random measurements of IAP alone. They also provide five sequential therapeutic steps to correct an abnormally low APP If one believes in the concept of transmural or perfusion pressure, as in the thorax or in the kidney that drives perfusion, it makes sound physiologic sense to follow such perfusion pressures (APP) and not absolute readings of pressure (IAP) alone.
Obviously, the proof of a physiologic concept, however sound, will rest in the clinical validation of the concept. Future studies, based on sound outcome measures, will determine the merit of this concept. Till then, it makes perfect sense to mark APP our most desirable goal.
References
1. Schein M, Ivatury R. Intra-abdominal hypertension and abdominal compartment syndrome. Br J Surg 1998; 85:1027-1028.
2. Bendahan J, Coetzee CJ, Papagianopoulos C et al. Abdominal compartment syndrome. J Trauma 1995; 38:152-153.
3. Schein M, Wittmann DH, Aprahamian CC et al. The abdominal compartment syndrome: The physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg 1995; 180:745-753.
4. Ivatury RR, Diebel L, Porter JM et al. Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin N Am 1997; 77:783-800.
5. Burch JM, Moore
EE,
G. Saggi BH, Sugerman HJ, Ivatury RR et al. Abdominal compartment syndrome. J Trauma 1998; 45:597-G09.
7. Cheatham ML. Intra-abdominal hypertension and abdominal compartment syndrome. New Horizons 1999; 7:9G-115.
8. Mayberry JC, Mullins RJ, Crass RA et al. Prevention of abdominal compartment syndrome by absorbable mesh prosthesis closure. Arch Surg 1997; 132:957-9G2.
9. Rotondo MF, Schwab W, McGonigal MD et al. Damage control: An approach for improved survival in penetrating abdominal trauma. J Trauma 1993; 35:375-382.
10. Chang MC, Miller PR, D'Agostino R et al. Effects of abdominal decompression on cardiopulmonary function and visceral perfusion in patients with intra-abdominal hypertension. J Trauma 1998; 44:440-445.
11. Ivatury RR, Porter JM, Simon RJ et al. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: Prophylaxis, incidence, and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J Trauma 1998; 44:1O1G-1023.
12. Sugrue M, Buist MD, Hourihan F et al. Prospective study of intra-abdominal hypertension and renal function after laparotomy. Br J Surg 1995; 82:235-238.
13. Cullen DJ, Coyle JP, Teplick R et al. Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med 1989; 17:118-121. 14. Sugrue M, Jones F, Jangua KJ et al. Temporary abdominal closure: A prospective evaluation of its effects on renal and respiratory physiology. J Trauma 1998; 45:914-921.
16, Malbrain MLNG, Chiumello D, Pelosi P et al. Prevalence of intra-abdominal hypertension in critically ill patients: A multicentre epidemiological study. Intensive Care Med 2004; 30:822-829. 17. Cheatham ML, Safcsak K, Llerena LE et al. Long-term physical, mental, and functional consequences of abdominal decompression. J Trauma 2004; SG:237-242.
18. Cheatham ML, White MW, Sagraves SG et al. Abdominal perfusion pressure: A superior parameter in the assessment of intra-abdominal hypertension. J Trauma 2000; 49:G21-G27.
19. Boulanger BR, Rapanos T, McLean RF et al. Intra-abdominal pressure in critically injured adults: Clinical assessment vs. bladder pressure. J Trauma 1997; 43:393.
20. Kron IL,
21. Cheatham ML, Safcsak K. Intraabdominal pressure: A revised method for measurement. J Am Coll Surg 1998; 186;594-595.
22. Malbrain MLNG. Abdominal pressure in the critically ill: Measurement and clinical relevance. Intensive Care Med 1999; 1453-1458.
23. Malbrain MLNG. Different techniques to measure intra-abdominal pressure (IAP): Time for a critical reappraisal. Intensive Care Med 2004; 30:357-371.
24. Malbrain MLNG. Abdominal perfusion
pressure as prognostic marker in intra-abdominal hypertension. In:
Vincent JL ed. Yearbook of Intensive Care and Emergency Medicine.
25. Coombs HC. The mechanism of the regulation of intra-abdominal pressure. Am J Physiol 1922; G1:159-170.
26.
27. Kashtan J, Green JF, Parson EQ et al. Hemodynamic effects of increased abdominal pressure. J Surg Res 1981; 30:249-255.
28. Diebel LN, Wilson RF, Dulchavsky SA et al. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma 1992; 33:279-282. 29. Ridings PC, Bloomfield GL, Blocher CR et al. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma 1995; 39:1071-1075.
30. Simon RJ, Friedlander MH, Ivatury RR et al. Hemorrhage lowers the threshold for intra-abdominal hypertension-induced pulmonary dysfunction. J Trauma 1997; 42:398-405.
31. Bradley SE, Bradley GP. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest 1947; 26:1010-1015.
33. Ulyatt DB. Elevated intta-abdominal pressure. Australian Anaes 1992; 108-114.
34.
35. Friedlander MH, Simon RJ, Ivatury R et al. Effect of hemorrhage on superior mesenteric artery flow during increased intra-abdominal pressures. J Trauma 1998; 45:433-439.
36. Diebel LN, Myers T, Dulchavsky S. Effects of increasing airway pressure and PEEP on the assessment of cardiac preload. J Trauma 1997; 42:585-591.
37. Diebel LN, Dulchavsky SA, Brown WJ. Splanchnic ischemia and bacterial translocation in the abdominal compartment syndrome. J Trauma 1997; 43:852-855.
38. Gargiulo NJ, Simon RJ, Leon W et al. Hemorrhage exacerabtes bacterial translocation at low levels of intra-abdominal pressure. Arch Surg 1998; 133:1351-1355.
39. Smith ER, Carter BS, Ogilvy CS. Proposed use of prophylactic decompressive craniectomy in poor-grade aneurismal subarachnoid hemorrhage patients presenting with associated large sylvian hematomas. Neurosurgery 2002; 51:117-124.
40. Cheatham ML, Safaak K, Block EFJ et al. Preload assessment in patients with an open abdomen. J Trauma 1999; 46:16-22,
41. Rotondo MF, Cheatham ML, Moore FA et al. Abdominal compartment syndrome. Contemp Surg 2003; 59:260-270.
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 5329
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2025 . All rights reserved