| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
Minerals:
Fe2+
Hemoglobin → O2 Transport
Supports electron transport chain → Complex III/IV
When decreased = Possible mental retardation in children
Ca2+
Necessary for muscle contraction
o All muscles need INTRACELLULAR Ca2+
o Cardiac & Smooth Muscle need EXTRACELLULAR Ca2+
Needed for atrial contraction
IP3/DAG Second messenger system
Mg2+
Co-factor for ALL KINASES and PTH
Cu2+
Need for the hydroxylation of lysine
Deficiency
o Minkys kinky hair
Orange hair
Feels like copper wiring
Excess = Wilsons Disease = hepatolenticular degeneration
o Lenticular → Basal ganglia
o
Trace
Elements: Chromium - neede
in Insulin action Selenium -
necessary for heart Manganese -
xanthine oxidase
Hepato → liver
o Keisher-fleisher rings in iris
o Ceruloplasmin deficiency
Zn
Hair, taste buds, dysgusia, sperm
Biochemistry:
CONCEPT:
Proteins:
Body is made up of mostly proteins.
o Recall that enzymes are proteins
But body likes to hang on to FAT (9 Kcal/1g)
SUGARS and AMINO ACIDS (4 kcal/ 1g)
Structure of Amino Acids: Exception: Proline:
![]()
![]()
![]() NH3
COOH NH2
COOH
NH3
COOH NH2
COOH
Amino Acid Imino
Imino group creates kinks
and bends Found in: hair,
muscle, skin, collagen, cartilage,
R
(determines structure of AA)
Buffers:
Proteins are the most important intracellular buffers
Bicarbonate is the most important extracellular buffer
Dissociation= loss of H+
Soluble = has charge and will attract H2O → Can not cross Blood Brain Barrier
Bioavailable = neutral, can cross a fat soluble membrane.
o When talking about Bioavailability think about Volume of distribution or t
Example:
NH3+ → NH2 = ↓ Solubility (by losing a charge); ↑ Bioavailability (by making it neutral)
COOH → COO- = ↑ Solubility (by adding charge); ↓ Bioavailability (b/c no longer neutral)
pKa:
1 2 3 4 5 6 7 8 9 10 11 14
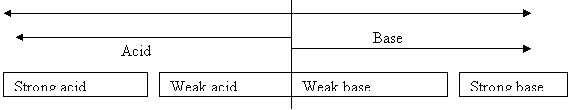
Titration Curve:
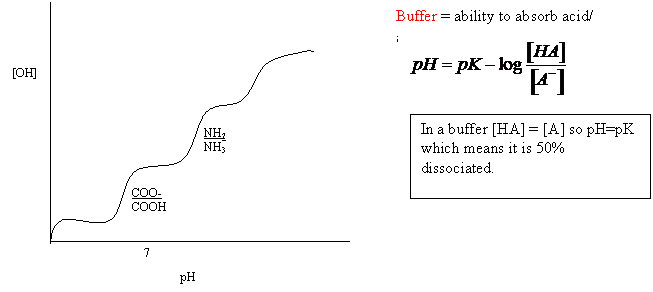
Histadine has pK = 6.7 which is closest to pH of 7.4 it is the best buffer in humans.
* Liver handles fat-soluble
content
Dissociation relationship:
pH = pK + 2 99% dissociated =99% soluble 1% bioavailable
pH = pK + 1 90% dissociated =90%soluble 10% bioavailable
pH = pK 50% dissociated Best Buffer
pH = pK - 1 10% dissociated =10% soluble 90% bioavailable
pH = pK - 2 1% dissociated =1% soluble 99% bioavailable
In order to absorb molecules they need to remain neutral = bioavailable. Follow these rules to keep molecules neutral.
To absorb more acid need to place in a stronger acid
o Acid + Strong Acid = Behaves as a BASE
To absorb more base place in a stronger base
o Base + Strong Base = Behaves as a ACID
Understand that the body makes 20x more HCO3- than acid
o Because we ingest primarily acidic substances
Example:
Common
Acids: ASA, Myoglobin (d/t crush
injury), phenobarbiltal. Common Bases:
Amphetamines
Common pHs:
Stomach pH = 1-2
Duodenum pH = 3-5
Early Jejunum pH = 5-7
Late Jejunum pH = 7-9
Ileum pH > 9
Example: ASA has pK = 4.3 (like other NSAIDs), therefore it would be absorbed best in stomach pH of 1-2, when 1% will be dissociated and 99% will be bioavailable for absorption.
Key Concept: Acid + Base will decrease absorption.
Example: When muscle breaks down releasing myoglobin, give bicarbonate to prevent secretion and further loss of myoglobin.
pI = pk1 + pK2 2
Isoelectric Point:
pI = Zwiterion = NO NET charge.
When you have more than two groups:
o like groups will have isoelectric point that will balance out opposite like group isoelectric point.
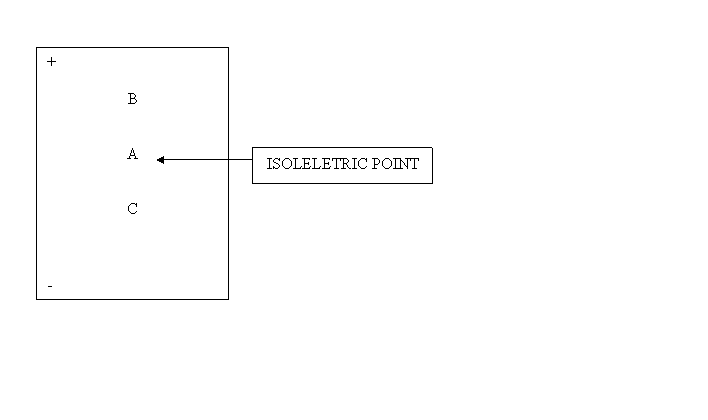
Gel Electrophoresis
Cathode is where cations GO
Anode is where anions GO
Amino Acids:
Groups
|
Amino Acid |
Abbreviation |
|
Alanine |
|
|
Glycine |
Gly |
|
Leucine |
Leu |
|
Proline |
Pro |
|
Threonine |
Thr |
|
Cysteine |
Cys |
|
Histidine |
His |
|
Isoleucine |
Ile |
|
Methionine |
Met |
|
Serine |
Ser |
|
Valine |
Val |
|
Arginine |
Arg |
|
Asparagine |
Asn |
|
Aspartate |
Asp |
|
Glutamate |
Glu |
|
Glutamine |
Gln |
|
Phenylalanine |
Phe |
|
Tyrosine |
Tyr |
|
Tryptophan |
Trp |
|
Lysine |
|
Acidic Groups = Asp, Glu
Basic Groups = Arg,
Sulfur = Cys, Met
O-Bonds = Ser, Thr, Trp
N-Bonds = Asp, Gln
Branched aa = Leu, Ile, Val
Bulky (aromatic) =Phe, Thr, Trp
Small =Gly
Kinky =Pro
Ketogenic =
(made and broken down to acetyl Co-A)
Glucogenic + Ketogenic = Phe, Iso, Thr, Trp
Glucogenic = All the rest.
GABA is a suppressor
causing: Bradycardia, Lethargy, Constipation, Impotence
GABA concept:
![]()
![]()
![]()
![]()
Essential Amino Acids:
Body will break down protein to look for essential amino acids if not provided by the diet.
PVT TIM HALL
Phenylalanine
Valine
Tryptophan
Threonine
Isoleucine
Methionine
Histidine
Arginine
Leucine
Always guess Autosomal recessive.
Lysine
Childhood screening: PKU, galactosemia, hypothyroidism, congenital adrenal
hypoplasia, biotindase.
Disorders:
Dopamine, Epinephrine and Norepinephrine mental retardation
Melanin for pigment pale, blond, blue eyes
Build up of phenylacetate + phenylpyruvate = musty odor
Screened in childhood GUTHIRE testing.
 Lysine
LysineProtein Structure:
L R R NCNC R Transfiguration
1 AA
sequence including the peptide bonds:
All Bonds are planar = flat
Restricted mobility
R groups face away from each other
2 a-helix vs. b- pleated sheet
Proline
Humans have L-amino
acids
will attack the D-amino acids Adipose layers have the least
amount of blood supply
will take longer to heal.
![]()
GI, vessels, hair flat bones, skin
3 3D- determined by:
Hydrophobic
Hydrophilic interactions.
Covalent bonds begin to form.
Ex. Hemoglobin
4 2
proteins interact with enzymes
Cooperativity
Allosterism (one site will affect another site)
HEMOGLOBIN
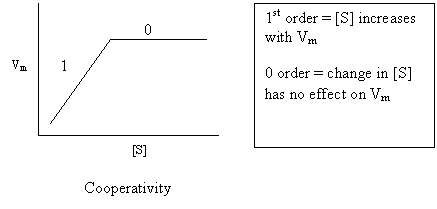
![]()
Vm [S] Allosterism
![]()
Meds: usually
exhibit 1st order kinetics Chemo drugs exhibit 0 order
kinetics Same amount of drug
metabolized over time regardless of concentration T
more toxic
Acid Hydrolysis:
o Glutamine glutamate
o Aspargine aspartate
Gel Electrophoresis:
o Smallest will move the farthest
o Then separate by charge.
Ninhydrin Reaction:
o Proline will stain yellow
o All others will stain purple.
Edmunds Degradation
o Will react with one amino acid at a time
o From the L amino terminal
o Used in spectrophotometry
o Good for only 100 amino acids.accuracy
Restriction Peptidase:
Amino Acids Sequencing
_ _ _ _ _
(lys, ala) (ser, met, phe)
You need to know which was amino acid was sequenced first!!!
If you cut with trypsin, where does it cut?
Trypsin cuts to the RIGHT of of lys and arginine!!!
_ lys/___ = Therefore, in a question, find the answer that already contains lys in the second position
KNOW WHERE THE ENZYME CUTS
Restriction Peptidases:
ALL CUT TO THE RIGHT
EXCEPTION:
Carboxy peptidase - cuts to the LEFT of any amino acid on carboxy terminal
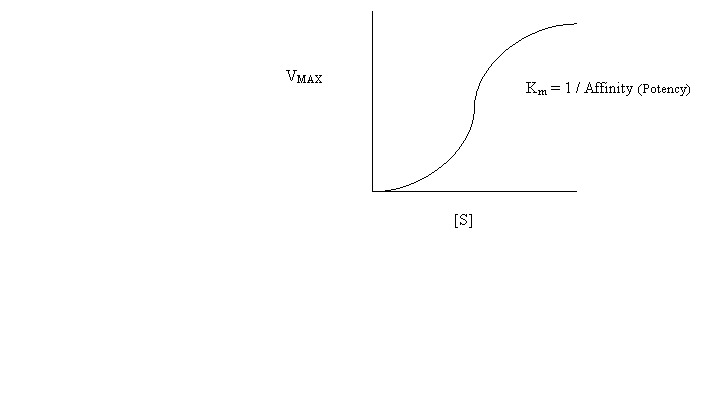 Allosterism
Allosterism
o Always the slowest
Inhibition:
o Competitive vs. Non-Competitive.
Competitive looks like substrate fights for active site
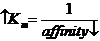
Vmax = Vmax
Non-competitive competing for regulatory site.
o No D in Km or affinity
o Vmax
Competitive vs. Non Competitive inhibitors:
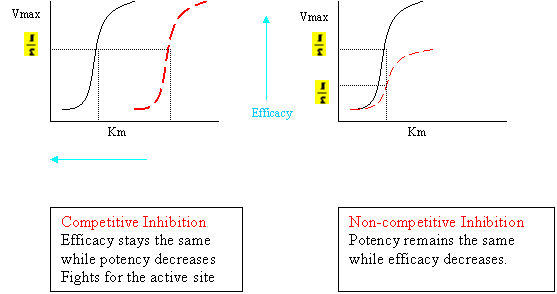
VMAX
VMAX
[S]
[S]
|
Km |
|
Km |
Hemoglobin:
|
Hg |
HbA 98% |
HbA2 2% |
HbF |
|
Genes |
a b |
a D |
a g |
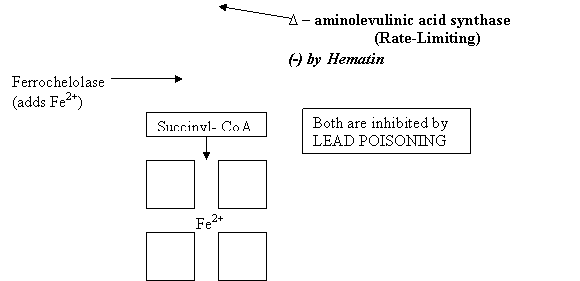
Anemias:
Microcytic Hypochromic anemia:
Sideroblastic Anemia:
Macrophages that eat iron:
o Parasitic infection
o Impaired iron absorption
o Liver disease (live stores Fe)
Porphyrias:
Enzyme deficiencies causing inability to break up heme → Degradation problem
Symptoms: Red urine indicating hemolytic anemia.
Acute Intermittent Porphyria (most common)
Recurrent acute abdominal pain and neuropathy (remember this can be anything..headaches, ↑ ICP, etc)
MCC = STRESS
o Can be set off by menses
MC Drugs that can cause this
o Sulfa
o Anti Malarial
o Metroniazole
o Barbituates
Treatment:
o Hematin stop Daminolevulinic acid synthase decrease further production of porphyrin.
o Fluids to flush it out
o Sugar helps draw the excess porphyrins out
Porphyria Cutanea Tarda
-Sun blisters skin
-Starts in late childhood > 5 years old
Erythrocytic Protoporphyria
-Early childhood < 1 years old
-Blister in the su
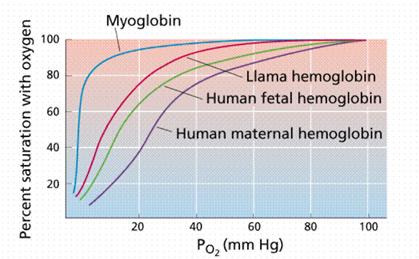 Myoglobin vs. Hemoglobin
Myoglobin vs. Hemoglobin
Fetal Hb:
Highest pO2 in umbilical vein coming from placenta (coming from mom)
o pO2 = 80
After liver pO2 to 60%
After brain pO2 to 50%
In extremities pO2 to 40%
Through Foramen Ovale and Left side pO2 = 90%
SaO2 = 90% pO2 = 60
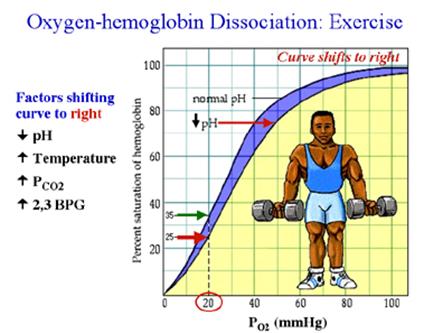 Hemoglobin
Hemoglobin
Normal values: Hg=15, Hct=45
1g of Hg has 4 Heme sites.
Athletes pO2 between 40
→ 60 = Hypoxic period Begin anaerobic →
↑ lactic acidosis The 2nd Wind Theory An athlete must out last
this hypoxic period so that Myoglobin can begin to drop oxygen
Ex;
If pO2< 60, free Hg to de saturate and curve shift to Right
Hg lets go of O2 and shift to Left Hg holding on to O2
o begins in the Yolk Sac at months gestation.
o 6 months liver, spleen and flat bones → close at 1 year
o 8 months long bones
o After 1 year of age long bones are in charge of erythropoesis.
If long bones are damaged, in bone marrow > 1 year spleen will take over again = splenomegaly
Inhibitors to Hb:
Carbons Monoxide:
Competitive inhibitor of O2
o Treatment = O2
Cyanide
o Non-competitive inhibitor of O2
o Km doesnt change
o Vmax will decrease
o Treatment with O2 will not make saturation go up
Hemaglobenopathies:
Sickle Cell Disease:
Autosomal recessive, HbS
Protect Against Malaria
Amino Acid substitution: Val Glu @ position 6 of b chain
o Valine = Neutral → goes inside
o Glutamic acid = Negative (charged) → goes outside
o THIS PROVIDES THE MECHANISM FOR SICKLING
When O2 decreases, the Val on opposite sides (positions 1 and 6) attract each other and change shape
SICKLE CELL = VASOOCLUSION
o Symptoms:
o Begin to feel cold, lightheaded, and experience syncope
o Dactylitis painful and swollen fingers and toes in new born
o Present at 4-6 months of age when Hg F switches to Hg S
o At 6 years → SPLENECTOMY
Sickle Cell Trait (SA)
Asymptomatic, but barred from extreme hypoxic situations or jobs
o Fireman, pilot, diver
Hg C:
Autosomal recessive
Amino Acid substitution:
o
o Glutamic acid (-) = outside
o BOTH STAY ON THE SURFACE = no sickling
Methemaglobanemia
Fe3+ cant pick up O2 = Ferric (oxidized)
o methemaglobanemia- inborn
o MCC = 2 methemaglobanemia- drug induced (sulfa) can oxidize Fe2+/Infections d/t free radicals
o Low O2 saturation BUT pO2 will be normal
Treatment:
o Methylene Blue Give them something blue to turn them pink
Anyl Nitrite- will convert Hg to Fe3+ not allowing CN to act.
Sodium Thiosulfate will bind CN and recant thiocyanate
Blood transfusion
Thalassemias:
Hg made up of:
o a subunit - 4 genes
o b subunit 2 genes
|
Thalassemia |
# of Genes missing |
% Hb Left |
Hb |
Symptoms |
|
α minor |
No symptoms |
|||
|
? symptoms |
||||
|
(+) symptoms, basophilic stippling |
||||
|
α major |
Hydrops Fetalis |
|||
|
β minor |
Always have ↑ HbA2 and HbF +/- symptoms based on lifestyle |
|||
|
β Major |
No HbA, asymptomatic until 6 mos. b/c time when HbF → HbA; All erythropoietic organs reopen |
Cooleys Anemia (Type of β Thalassemia)
Ineffective erythropoiesis → making useless RBC
Baby making blood from everywhere:
o Frontal Bossing
o Large sternum/ clavicles
o Hepatosplenomegaly
o Long tender extremities
o HCT ↑↑↑, but Hb ↓↓↓
Treatment:
o Total body transfusion every 60-90 days → TRANSFUSION DEPENDENT
o Recall that a RBC only lives 120 days
o 1 unit of PRBC =
Hg by 1-2g
Fe by 3-4g
Will die within I 10 years of transfusion related infections
Can die d/t Iron overload = Hemochromatosis
Hemosiderosis:
o Bone marrow overwhelmed with Fe due to frequent transfusions.
o
Transfusion
Infections Hep B Hep C HIV CMV EBV Hep D Malaria Bacteria Babesosis Syphuliis
Sideroblastic anemia
Hemochromatosis: Deposit Fe into organs.
10 Hemochromatosis:
o Congenital rare autosomal recessive, HLA3 + Chr 6
o Duodenum absorbing too much Fe leading to:
Hemosiderosis
Hemochromatosis
Hemochromatosis:
o Acquired
o Due to transfusions:
Bronzing accumulates in skin
o Will die of:
1st decade of life transfusion related infections
2nd decade of life HF
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 1121
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2026 . All rights reserved