| CATEGORII DOCUMENTE |
| Bulgara | Ceha slovaca | Croata | Engleza | Estona | Finlandeza | Franceza |
| Germana | Italiana | Letona | Lituaniana | Maghiara | Olandeza | Poloneza |
| Sarba | Slovena | Spaniola | Suedeza | Turca | Ucraineana |
Effect of Ultrasound, Temperature and Pressure Treatments on Enzyme Activity and Quality Indicators of Fruit and Vegetable Juices
Abstract
The most heat stable enzyme in lemon and other citrus juices is pectinesterase (PE). This enzyme induces pectin destabilization, which causes cloud loss in the juice. The cloud presents the fresh-like property and therefore product satisfaction. Inactivation of PE is generally used as an indicator of the adequacy of pasteurization because it is known to be more heat resistant than the common micro-organisms. Ultrasonic treatment is one of the emerging tools that could be the alternative to thermal processing. It can enhance convective heat transfer as well as generate bubble explosion, which produce local hot spot that can cause micro-organism and enzyme destruction. However, ultrasonication (US) alone cannot inactivate the thermo-stable PE, even at long exposures. The combination of ultrasound and heat (thermosonication, TS) can slightly decrease the activity of this enzyme, which depended on time and temperature. Manothermosonication (MTS) is a method of combining ultrasonication with thermal and pressure treatment. This method can significantly decrease the activity of PE at the moderate pressure (100-300 kPa) of temperature below 100C. Almost complete enzyme inactivation (94% inactivation at 70C, 300 kPa, 2 min and 96% inactivation at 80C, 200 kPa, 5 min) occurred under the conditions mentioned. The extent of inactivation depended on pH, time of exposure, temperature, pressure and amplitude of the ultrasound. Lowering the pH of the medium increased the inactivation of the enzyme. PE activity of 0.55 unit/ml was obtained at pH 2.5, 30C, 1 min whereas 2.5 unit/ml was obtained at pH 7.5, 30C, 1 min. Increase in time of exposure (46% increased inactivation from 3 min to 38 min at 70C, 65% increased inactivation from 3 min to 63 min at 80C), temperature (for 3 min treatment time; 3.7% inactivation at 40C, 95% inactivation at 80C, 98% inactivation at
90C), pressure (at 70C, 2 min PE was 26% inactivation at 100 kPa and 35% inactivation at 300 kPa) and ultrasonic power (at 80C, 300 kPa, PE was 83.5% inactivation at ultrasonic power of 20%, 87% inactivation at power of 50% and 91% inactivation at power of 100%) enhanced enzyme inactivation. The improvement of the inactivation can be represented by pressure enhancing US (e.g. for MTS inactivation of PE, D60(100 kPa) = 3.6 min, D60(300 kPa) = 1.18 min), US enhancing heat and pressure (e.g. for the experiment o f MTS inactivation of PE in fresh lemon juice at 75C, 300 kPa, 60% inactivation without ultrasound, 77% inactivation at 20% ultrasonic power, 81% inactivation at 50% ultrasonic power) and temperature-pressure treatment inducing chemical reactions. The decimal reduction times of MTS inactivation of tomato were also dramatically decreased. For pectinesterase, D-value of 7.39 min at 60C, 400 kPa, 100% ultrasonic power was obtained where D-value of 21 min was obtained at 60C without MTS treatment. The same phenomenon was observed in polygalacturonase (D = 12.74 min, D60(MTS) = 5.63 min) peroxidase (D = 21 min, D60(MTS) = 7.4 min) and polyphenoloxidase (D = 14.7 min, D60(MTS) = 8.9 min) inactivation. These inactivations depended on temperature (e.g. PE; D = 12.8 min, D = 1 min) and time (e.g. PE activity at 70C, 1 min = 0.49 unit/ml, PE activity at 70C, 5 min = 0.08 unit/ml). Further investigation should focus on the mechanisms of the combination treatment. MTS treatment was also investigated on fresh lemon juice and strawberry juice. It has been shown the great potential of this new technology since the MTS treatment could maintain properties such as cloud, colour, pH and conductivity. However, in terms of the nutrition value, ascorbic acid undergoes degradation during the treatment and storage. One needs to investigate further on the optimum treatment of MTS (e.g. oxygen removal) in order to preserve the nutritional indicators in lemon juice.
Abbreviations
a -colour tone (red and green)
b -colour tone (yellow and blue)
A -Enzyme activity (unit/ml)
Ai -The initial activity of the enzyme (unit/ml)
Af -The residual activity of the enzyme (unit/ml)
B -The frequency or Arrhenius factor
C -Constant term, equal to kBT/h
D -Decimal reduction time (min)
DT -Decimal reduction time at temperature T (min)
DrefT -Decimal reduction time at reference temperature (min)
Ea -Arrhenius activation energy (kJ/mol)
EI -Inactive enzyme
EN -Native or active enzyme
EN* -Transition state enzyme
h -Constant of Planck (6.262 Js)
HP -High pressure treatment
k -Specific rate constant (min
kB -Boltzmann constant (1.38 J/K)
krefP -Inactivation rate constant at reference pressure (min
krefT -Inactivation rate constant at reference temperature (min
K* '-Quasi-equilibrium' constant
MS -Manosonication
MTS -Manothermosonication
n -The order of reaction
pD -The monitored flowrate in the pilot plant (l/h)
pS -The monitored pressure in the pilot plant (kPa)
P -Pressure treatment
P -Treatment pressure (kPa)
Pref -Reference pressure (kPa)
PE -Pectinesterase
PG -Polygalacturonase
POD -Peroxidase
PPO -Polyphenoloxidase
PS -Presonication
PTS -Postsonication
R -Gas constant (J/mol K)
t -treatment time (min)
T -Thermal treatment
T -Treatment temperature (C)
Tref -Reference temperature (C)
TS -Thermosonication
US -Ultrasonication or ultrasonic amplitude
Va -Activation volume (cm /mol)
z -Temperature dependence (C)
Introduction
Chapter 1
General consideration and aims
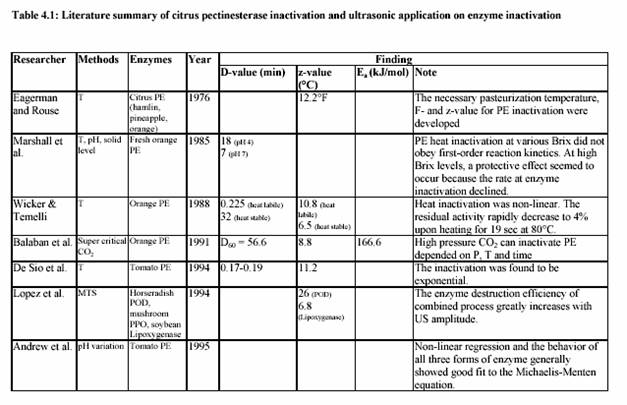
Commercial lime and lemon juices are among the world most important citrus products. For example, these juices are used as a common ingredient in most of the traditional Asian cooking. Moreover, they are necessary for many global food productions, such as lemonade drink, marmalade, jams, candies, jellies, desserts, pharmaceutical products, and medicines. Lemon juice, itself, is a colloidal suspension of cellular and polymer particles. This cloudy appearance is an important property of the juices since it gives the natural appeal of the fresh juices. Colloidal stability is maintained by pectin molecules through a complex and not well understood mechanism. Cloud loss of citrus juices is an intensively studied problem in food technology. It is due to the action of endogenous pectinesterase (PE) on pectin substance. PE catalyzes the de-esterification of pectin molecules. De-esterified pectin molecules are able to interact through calcium bridges, leading to cloud loss and phase separation in single-strength lemon juices and gelation in their concentrates. Stabilization of cloud in citrus juices requires the inactivation or inhibition of PE (Vercet, et al., 1999). Several strategies have been used to inhibit or inactivate PE avoiding the negative effects of intensive heat treatments. Inhibition of PE by polyphenols (Hall, 1966; Pilnik and Voragen, 1991), inhibition by specific proteic PE inhibitors (Castaldo et al., 1991), or inhibition by the oligogalacturonides produced by the action of added polygalacturonase or pectinylase (Baker and Bruemmer, 1972; Krop and Pilnik, 1974; Termote et al., 1977) have been suggested as alternatives to the heat treatments. Other strategies rely on PE inactivation by nonthermal treatments such as high pressure (Irwe and Olson, 1994; Donsi et al., 1996; Cano et al., 1997; Knorr, 1998), low pH values (Owusu-yaw et al., 1988), or supercritical carbon dioxide (Arreola et al., 1991; Balaban et al., 1991; Ishikawa at al., 1996).
Another possible alternative is ultrasound in combination with heat and pressure (Manothermosonication; MTS). MTS is an emerging technology that efficiently combines the inactivating effect of heat and ultrasonic waves (Burgos, 1998). MTS has been proved to be an efficient tool to inactivate some other enzymes, such as lipoxygenase, peroxidase, and proteases and lipases from psychrophic bacteria (Lopez at al., 1994; Sala at al., 1995; Vercet at al., 1997). Most results reported in the scientific literature, in fact, relate deactivating and destructive action of ultrasound only to its frequency and fail to provide information about the dependence of the treatment efficiency on the actual power and power density of ultrasound. In addition, no definite experimental evidence has been reported on the efficiency of ultrasound in batch or in continuous applications. The aims of the study were to investigate the effect of ultrasound on the inactivation
of lemon pectinesterase and juice quality. One further aim was the evaluation of the kinetic parameters of the MTS effect on lemon and tomato pectinesterase. Finally, the potential of the MTS in fruit and vegetable (lemon, strawberry, tomato) juice industry was determined.
Chapter 2
Ultrasonic science
2.1 Sound ranges
The range of human hearing is from about 16 Hz to 18 kHz. Ultrasound is the name given to sound waves having frequencies higher than those to which the human ear can respond (i.e. > 18 kHz). The upper limit of ultrasound frequency is one, which is not sharply defined but is usually taken to be 5 MHz in gases and 500 MHz in liquids and solids. The use of ultrasound within this large frequency range may be divided broadly into two areas. The first area involves low amplitude (higher frequency) propagation, which is concerned with the effect of the medium on the wave and is commonly referred to as low power or high frequency ultrasound. Typically, low amplitude waves are used to measure the velocity and absorption coefficient of the wave in a medium in the 2 to 10 MHz range. It is used in medical scanning, chemical analysis and the study of relaxation phenomena. The second area involves high energy (low frequency) waves known as power ultrasound between 20 and 100 kHz which is used for cleaning, plastic welding and, more recently, to effect chemical reactivity.
Ultrasonic waves are generated by mechanical vibrations of frequencies higher than 18 kHz. When these waves propagate into liquid media, alternating compression and expansion cycles are produced. During the expansion cycle, high intensity ultrasonic waves make small bubbles grow in liquid. When they attain a volume at which they can no longer absorb enough energy, they implode violently. This phenomenon is known as cavitation.
During implosion, very high temperatures (approximately 5000 K) and pressures (estimated at 50000 kPa) are reached inside these bubbles (Sala et al., 1998).
2.2 Mechanisms and effects
High-intensity acoustic radiation causes various changes as is propagates through a Medi um. These changes can be explained by several mechanisms, but not all mechanisms involved are known or well understood. Most of the reported effects and proposed mechanisms can be summarized as follows:
Heating: As a result of specific absorption of acoustic energy by membranes and biomaterials, particularly at their interfaces, a selective temperature increase may take place (Floros and Liang, 1994). This heating effect was assumed to be responsible for the significant increase in diffusion of sodium ions through living frog skin under ultrasound (Lehmann and Krusen, 1954). The increase in permeability of the living membrane was so large that its selectivity was completely lost. Later theoretical and experimental results do not support the early assumptions. Floros and Liang (1994) emphasized about the heat balance equation to calculate loss of ultrasonic energy as it propagates through a medium. They mentioned that the temperature change due to absorption at a solid wall, under given conditions, was 0.1 C for water and about 1 C for air. These results were verified
experimentally. Other investigators claim that localized temperature increase of up to 5000 K can be expected for a few nanoseconds in a sound field (Suslick et al., 1985).
Cavitation: Acoustic cavitation is the formation, growth, and violent collapse of small bubbles or voids in liquids as a result of pressure fluctuation (Suslick, 1988). In general, cavitation in liquids may cause fast and complete degassing; initiate various reactions by generating free chemical ions (radicals); accelerate chemical reactions by facilitating the mixing of reactants; enhance polymerization/ depolymerization reactions by temporarily dispersing aggregates or by permanently breaking chemical bonds in polymeric chains; increase emulsification rates; improve diffusion rates; produce highly concentrated emulsions or uniform dispersions of particles; assist the extraction of substances such as enzymes from animal, plant, yeast, or bacterial cells; remove viruses from infected tissue; and finally, erode and break down susceptible particles, including micro-organisms.
Structural effects: When flu ids are placed under high intensity sound fields, the dynamic agitation and shear stresses produced affect their structural properties, particularly their viscosity. Usually, Newtonian fluids maintain their characteristics, but dilatants and thixotropic fluids tend to either stiffen or become less viscous, respectively (Ensminger, 1986).
Compression and rarefaction: When high-intensity acoustic energy travels through a solid medium, the sound wave causes a series of rapid and successive compression and rarefaction, with rates depending on its frequency. In turn, the material is subjected to a rapid series of alternating contractions and expansions, much like when a sponge is squeezed and released repeatedly. This mechanism, known as rectified diffusion, is very important in acoustic drying and dewatering and noticeable moisture migration takes place overall (Ensminger, 1986). In more dense materials that are practically incompressible, the alternating acoustic stress facilitates dewatering by either
maintaining existing channels for water movement or creating new ones. Dense materials usually fracture under acoustic stress. Microscopic channels are created in directions normal to wave propagation during rarefaction, or parallel to wave propagation during compression (Floros and Liang, 1994). The same mechanism results in elevated and reduced pressure at gas/liquid interfaces, and therefore increases evaporation rates. Although the pressure variation introduced by the sound wave is very low, its effect is strong because of the rapid rate of pressure oscillation.
Turbulence: High-intensity ultrasound in low-viscosity liquids and gases produces violent agitation, which can be utilized to disperse particles (Ensminger, 1988). At liquid/solid or gas/solid interfaces, acoustic waves cause extreme turbulence known as acoustic streaming or micro streaming (Nyborg, 1965). This reduces the diffusion boundary layer, increases the convection mass transfer, and considerably accelerates diffusion in systems where ordinary mixing is not possible.
Other: A number of other effects and mechanisms have been reported. Ultrasonic waves of high intensity assist the cleaning of surfaces. This mechanism has been used to prevent binding or formation of filter cake and enhance filtration rates (Floros and Liang, 1994). Under certain conditions, high-intensity ultrasound causes coalescence of many types of particles and can be used effectively in low-concentration suspensions.
Chapter 3
Effect of ultrasound on
enzyme inactivation
3.1 Inactivation kinetics of enzymes
At a constant pH, degradation of nutrients and inactivation of enzymes and microorganisms are usually described by first-order rate expressions. Temperature effects on these reactions usually agree with the Arrhenius equation. At different pH values these reactions occur via different mechanisms, so the activation energy and frequency factor in the Arrhenius equation vary with pH in a complex manner (Uelgen and Oezilgen, 1993).
Response surface methodology is preferred for optimization of such complex processes. In this technique dependent variables (fraction of the surviving enzyme activity, logarithm of the surviving microbial population and fraction of the vitamin retained) are described as arbitrary functions of the independent variables (pH of the juice, processing time and processing temperature). A sufficiently large number of terms are included for these functions to mirror the variations in the experimental data very closely.
3.1.1 Evaluation of D and z values of enzyme for thermal process
calculation
As in thermobacteriology, enzymatic inactivation features kinetic parameters that make it possible to compare the degree of inactivation and the thermal treatment defined by the temperature-time curve. The most important kinetic parameter is the decimal reduction time (DT) and its dependence on temperature expressed by z. The decimal reduction time was calculated according to Stumbo (1973) by equation:
DT = t/(log Ai - log Af
where Ai is the starting activity; Af is the residual activity that survives the thermal treatment; and t is the thermal treatment time at temperature T in minutes. The z parameter was derived from log DT values at different treatment time versus temperature. The z parameter indicates how many degrees the temperature must change for the decimal reduction time to be 10 fold higher or lower.
z = -(T - Tref)/(log D - log DrefT
where T = Temperature in C (or K) for the lower temperature
Tref = Temperature in C (or K) for the higher temperature
D D-value for the lower temperature (min)
DrefT D-value for the higher temperature (min)
3.1.2 Order of the reaction
Protein and enzyme denaturation often follows first-order kinetics; by definition that a single molecule undergoes a conformational change. However, when more than a single kind of enzyme is present, as is frequently the case in food enzyme preparations, the kinetics may be complex.
If first-order kinetics can be assumed, the process is
EN EI
where EN is the native or active enzyme, EI is the inactive enzyme, and k is the specific rate constant for the inactivation process. Mathematically the decrease in active enzyme becomes
-d[EN]/dt = k[EN or -dA/dt = kA (3.4)
where A is enzyme activity, then integration gives
ln([EN]/[ENi]) = -kt or ln(A/Ai) = -kt (3.5)
where ENi is the initial native, active enzyme and Ai is initial enzyme activity.
3.1.3 Temperature dependence
The most commonly used mathematical expression for the effect of temperature on rates of chemical processes, including enzyme reactions, is Arrhenius relationship,
k = B exp-Ea/RT
ln k = ln B - Ea/RT (3.7)
where B is the frequency or Arrhenius factor and Ea is the Arrhenius activation energy. Equation 3.7 indicates a linear relationship when ln k is plotted against 1/T. The slope of this plot is -Ea/R. The magnitude of Ea indicates the temperature dependence of the reaction in question.
For a definition of reference temperature, equation 3.17 can be rewritten as
ln k = ln krefT
where krefT = inactivation rate constant at Tref (min
Tref = reference temperature (K)
3.1.4 Transition state theory and pressure dependence
The similarity between the Arrhenius and Vant Hoff equations should be noted. However, Ea is not identical to enthalpy ( H Yet, the similarity between the two equations suggests that values for Ea may provide some insight into the thermodynamic nature of chemical processes. The transition state, or Eyring theory, is the most successful attempt to relate irreversible kinetic information to thermodynamic information. In brief, this theory suggests that native enzyme EN , in an irreversible reaction, goes through a transition state EN* which is in equilibrium with the native enzyme.
EN EN* EI
where K* = quasi-equilibrium constant
The K* value does not really represent an equilibrium but rather the probability that reactants will get into the activated state and decompose. The rate of disappearance of EN is given by
-dEN/dt = C[EN*
where C = constant term, equal to kBT/h
kB = Boltzmann constant (1.38 J/K)
h = constant of Planck (6.262 Js)
Since K* [EN*]/[EN eqn. 3.10 becomes
-dEN/dt = k[EN
where k = CK* = the specific rate constant for the transition process
From G* = -RTlnK* , then k can be derived as follows:
k = (kBT/h)exp(- G*/RT) (3.12)
The dependence of the inactivation rate constant on pressure can be given as
follows:
ln k = ln K* (3.13)
P P
-RT ln k = -RT ln K* = G* = V* (3.14)
P P P
where V* is activation volume (cm /mol). From this equation, it can be derived that inactivation is enhanced by pressure if the volume decreases. For the reference rate constant at reference pressure, the equation 3.14 can be derived as
ln k = ln krefP - [Va/RT(P-Pref
where krefP = inactivation rate constant at Tref (min
Pref = reference pressure (kPa)
3.2 Application of ultrasound on enzyme inactivation
3.2.1 General information
It has been known for many years that ultrasound can be employed as a method of inhibiting enzyme activity. Peroxidase, which is found in most raw and unblanched fruit and vegetables, is particularly associated with the development of off-flavours and browning pigments. The original activity of peroxidase was progressively reduced by 90% as ultrasound was applied over a 3 h period. Nearly 60 years ago Chambers reported that pure pepsin was inactivated by sonification probably as a result of cavitation. The implosion of internal cavitating bubbles induced by ultrasound waves generates microscopically small hot spots (temperatures estimated at 5000 K) and local pressures of 50000 kPa (Suslick, 1988). In MTS, moderate pressure is applied to allow cavitation at temperatures close to or above the boiling point. This also increases the implosion intensity, which is related to the difference between the static pressure and the vapour pressure inside the bubble (Vercet, 1999).
The introduction of refrigerated tanks for bulk storage of milk before heat treatment has reduced spoilage by mesophilic micro-organisms but favours the growth of psychrotropic bacteria. Although these micro-organisms are easily destroyed under standard heat treatments, many of them produce extracellular lipase and protease, which withstand UHT treatment (Stead, 1986). These thermoresistant enzymes can reduce the quality and shelf-life of heat-treated milk and other dairy products. The simultaneous application of heat and ultrasound waves under pressure (manothermosonication; MTS) has also been found to be more effective than heat treatment in the inactivation of these heat resistant protease and lipase secreted by P. fluorescens (Vercet et al., 1997).
3.2.2 Application of ultrasound
3.2.2.1 Ultrasonication
Ultrasonication (US) is operated at low temperature. Therefore, a product with heatsensible
components can be treated. However, the treatment time is actually long during the inactivation of enzymes and/or micro-organisms, which may cause high-energy requirement. Normally, this treatment will need to be combined with other techniques to optimize the process.
3.2.2.2 Presonication
In case of presonication (PS), the product is pretreated by ultrasound before subjected to the
heat and/or pressure treatment. In this case, the enzymes and/or micro-organisms are
resistant to heat and pressure. Pre-treatment with ultrasound can minimize the resistance of
enzymes and micro-organisms, which can be completely inactivated by the following
temperature and pressure treatment.
3.2.2.3 Thermosonication
In thermosonication method (TS), the product is subjected to ultrasound and moderate heat
simultaneously. This technique shows the same inactivation level compared to the treatment without ultrasound at high temperature. The temperature, however, is rising during the treatment. Therefore temperature control is required.
3.2.2.4 Postsonication
By applying postsonication (PTS), the product is treated with heat and/or pressure before being subjected to ultrasound. This technique is still not popular and there are no experimental data available on this technique so far.
3.2.2.5 Manosonication
Manosonication (MS) provides the possibility to inactivate enzymes and/or microorganisms
by combining ultrasound with moderate pressure 100 - 300 kPa at low temperature.
3.2.2.6 Manothermosonication
Manothermosonication (MTS) combines the ultrasound with moderate temperature and moderate pressure in order to inactivate enzymes and/or micro-organisms. The ultrasound generates the cavitation or bubble implosion in the media. This implosion can cause inactivation of enzyme and destruction of micro-organisms. The simultaneous pressure treatment maximizes the intensity of the explosion, which increases the level of inactivation.
Chapter 4
Application of ultrasound
in food industry
4.1 General information about ultrasound in food industry
There is presently much industrial interest in developing mild food preservation procedures, which could replace the severe heat-based methods, which are currently in common use. Often termed minimal processing, the benefits of these approaches are an important aspect of current and future commercial product development. Quality attributes, which can be protected by application of minimal process technologies, are: flavour and odour, visual appearance, i.e. colour and texture, nutrition value, absence of additives.
Minimal processing can be applied to a wide variety of foods including short shelf-life products such as fresh fruit and vegetables, chilled ingredients and convenience dishes through to long-life ambient stable foods such as cooked meats and vegetables. This commercial challenge has opened up new opportunities for combined preservation systems incorporating mild heat treatments or low food additives and provided impetus for the new variables for microbial control.
The use of ultrasound within the food industry has been a subject of research and development for many years and, as is the case in other areas, the sound ranges employed can be divided basically into high frequency, low energy, diagnostic ultrasound in the MHz range and low frequency, high energy, power ultrasound in the kHz range.
Application of ultrasound in food processing can be classified into two main categories:
1. monitor a process or product
2. affect a process or product
4.1.1 Diagnostic ultrasound
Up to a few years ago the majority of applications and developments involved non-invasive
analysis with particular reference to food quality assessment, e.g. by monitoring the
attenuation of an ultrasound pulse it has been proven possible to determine the degree of
homogenization of fat within milk. Several reports have summarized, classified, and
critically reviewed the present and future outlook of low-intensity, high frequency
ultrasound for process/product monitoring (Floros and Liang, 1994). Useful industrial
applications include texture, viscosity, and concentration measurements of many solid or
liquid foods; composition determination of eggs, meats, fruits and vegetables, dairy, and
other products; thickness, flow, and temperature measurements for monitoring and control
of several processes; and non-destructive inspection of egg shells and food packages.
4.1.2 Power ultrasound
Application of ultrasound to directly improve processes and products is less popular in food
manufacturing, but well recognized in other industries. High-intensity sound is mainly used
for such applications with frequency either in the sonic (< 18 kHz) or ultrasonic ( 18 kHz)
range, depending on the application. The beneficial use of sound is realized through its
chemical, mechanical, or physical effects on the process or product. In fact, a new branch
of chemistry called sonochemistry has been created to take advantage of the chemical effects of ultrasound (Suslick, 1986).
4.1.2.1 Acceleration of the reaction
General applications include acceleration of conventional and decomposition reactions, degradation of polymers, and polymerization reactions (Floros and Liang, 1994). When particles of material in a liquid suspension are subjected to sonication a number of physical and mechanical effects can result. Large particles are subject to surface erosion (via cavitation collapse in the surrounding liquid) or particle size reduction (due to fission through interparticle collision or the collapse of cavitation bubbles formed on the surface). Recent studies on the effect of sonication on suspended powders have shown that the particles can be forced into violent collision that, in the case of metals, fusion can occur. In some cases the colloding powders undergo chemical reaction. Thus when copper and sulphur are sonicated together in hexane for 1 hour, 65% Cu S is generated (Goh et al.,
4.1.2.2 Cleaning and degassing liquids
The mechanical and physical effects of sound are utilized to improve cleaning of surfaces (Crawford, 1963). The cavitational effects, which are the basis of sonochemistry, are also the reason for the extremely effective uses of ultrasound for the degassing of liquids. Any dissolved gases or gas bubbles in the medium act as nuclei for the formation of cavitation bubbles. Such bubbles are not easily collapsed in the compression cycle of the wave due to the fact that they contain gas and they will continue to grow on further rarefaction cycles, filling with more gas and eventually floating to the surface. Since the rarefaction cycles are taking place extremely rapidly (around 40,000 times per second using an ultrasonic bath) the bubbles grow so quickly that degassing appears to occur almost instantaneously.
4.1.2.3 Crystallization
Power ultrasound has proved to be extremely useful in crystallization processes (Mason et al., 1996). It serves a number of roles in the initiation of seeding and subsequent crystal formation and growth. It also has a secondary property, which is beneficial in such processing applications, namely that the cleaning action of the cavitation effectively and thereby ensures continuous efficient heat transfer. It has been reported that ultrasound can be used to clarify wines through the precipitation of potassium bi-tartrate (Mason et al., 1996). The texture of food products will be affected by the size of undissolved sugar crystals dispersed in the material. Size will also affect the rate of dissolution of sugar in food preparation. For these reasons the control of sugar crystallite size is important. Normal crystallization of sugar from concentrated sucrose solutions leads to large uneven sized crystals, which can be broken down by subsequent sonication (Mason et al., 1996). One
very important area related to crystallization in the food industry is the formation of ice crystals during freezing of water.
4.1.2.4 Drying and filtration
The requirement to remove suspensions of solids from liquids is common to many industries including chemical, engineering as well as food. This separation can be either for the production of solid-free liquid or to isolate the solid from its mother liquors. Ultrasonic filtration of particulate matter from a liquid is now arousing some interest since the rate of flow through a filter can be increased substantially on application of ultrasound. There are two specific effects of ultrasonic irradiation which can be harnessed to improve the filtration technique: (1) sonication will cause agglomeration of fine particles and lead to rapid filtration, which (2) will supply sufficient vibrational energy to the system to keep the particles partly suspended and therefore leave more free channels for solvent elution. The combined influence of these effects has been successfully employed to enhance vacuum filtration of industrial mixtures such as coal slurry, which is a particularly time consuming and difficult process (Senapati, 1991).
4.1.2.5 Inactivate micro-organisms and enzymes
Food preservation by elevated temperature for short period of time is still the most common form of food preservation process. In most cases the process variables and controls have been derived by empirical investigation of the effect of temperature and time of exposure on microbial survival kinetics with little regard given to food quality in relation to thermal effects on food composition and structure. The loss of quality is brought about by deformation of plant and animal structures, modification of macromolecules and the production of new substances from heat-catalyzed reactions. Ultrasound offers some exciting opportunities to reduce these effects. The high temperatures to which microbial cells are exposed will not affect one specific target, as the thermal energy in the cell is an integral part of an entire complex system. The heat energy affects a wide variety of cellular constituents, including structures, molecules and the reactions in the cell. These targets provide opportunities to harness the cell damaging ability of ultrasound for combined
preservation systems.
The application of ultrasonic waves generating cavitation in suspensions, which contain micro-organisms and enzymes, often has a lethal result and deactivating action (Suslick, 1988). When high power ultrasound propagates into a liquid the micro-bubbles, which are commonly present in it or that may form from the presence of suspended particles, will oscillate according to the pressure wave. High acoustic pressure will determine their growth and violent collapse, which is accompanied by a sudden increase of the temperature and the pressure in the surrounding area.
4.1.2.6 Effect on rice grains If the particles subjected to sonication are rice grains in water then some destruction of surface shell and grain fragmentation would be anticipated. Both of these effects would result in a faster release of starch during cooking leading to a shorter period to form a gel (Mason et al., 1996).
4.1.2.7 Accelerate extraction processes
Ultrasound assists extraction processes. The classical techniques for solvent extraction of materials from vegetable sources are based upon the correct choice of solvent coupled with the use of heat and/or agitation. The extraction of organic compounds contained within the body of plants and seeds by a solvent is significantly improved by the use of power ultrasound. The mechanical effects of ultrasound provide a greater penetration of solvent into cellular materials and improve mass transfer. There is an additional benefit for the use of power ultrasound in extractive processes, which results from the disruption of biological cell walls to facilitate the release of contents. This has been shown in a study from sugar beet (Mason et al., 1996). Chymosin and some other enzymes soluble in sodium chloride solution are extracted from calf abomasa. The current commercial extraction process has been improved to maximize recovery of chymosin and minimize presence of other protease and lipase (Barbono, 1986). Ultrasound may be the simplest and most versatile method of breaking cells and preparing extracts. The release of enzymes and proteins from cells and
subcellular particles is a unique and effective application of ultrasound, which causes destruction of cellular structure by a cavitation effect (Kim and Zayas, 1991).
Protein extraction from defatted soya beans was studied by Povey and Mason (1998). A continuous process was developed where sonification of the slurry by a 550 W probe operating at 20 kHz frequencies resulted in an efficient extraction which exceeded any previously available technology. This was scaled up to pilot plant level for the extraction of soya bean protein (Povey and Mason, 1998).
4.1.2.8 Meat products
Closely linked with extraction is the methodology employed for the production of meats. Generally this involves tumbling the meat particles with an aqueous liquor containing salt. Ultrasound assists the process by disrupting the meat myofibrils, which releases sticky exudates, and this binds the meat together and leads to an increase in the strength of the reformed product. The binding strength, water holding capacity, product colour and yields were examined after treatment either with salt tumbling, sonication or both. Samples, which received both salt treatment and sonication, were superior in all relevant quality. A study of the effect of sonication on cured rolled ham showed similar result. A traditional method of tenderisation of meat is by mechanical pounding, which makes poor quality meat more palatable. Sonication of steak has also been found to be useful in the tenderisation process.
4.1.2.9 Emulsification
One of the earliest uses of power ultrasound in processing was in emulsification (Povey and Mason, 1998). If a bubble collapses near the phase boundary of two immiscible liquids the resultant shock wave can provide a very efficient mixing of the layers. Stable emulsions generated with ultrasound have been used in the textile, cosmetic, pharmaceutical and food industries. Such emulsions are often more stable than those produced conventionally and often require little, if any, surfactant. Emulsions with smaller droplet sizes within a narrow size distribution are obtained, when compared to other methods. The degree of emulsification in such materials can also be estimated by the measurement of ultrasound velocity in conjunction with attenuation. It is possible to determine factors such as the degree of creaming (or settling) of a sample, i.e. the movement of solid particles/fat droplets to the surface (or to the base). Such information gives details, for example, of the long-term stability of fruit juices and the stability of emulsions such as mayonnaise. The
combination of velocity and attenuation measurements shows promise as a method for the
analysis of edible fats and oils and for the determination of the extent of crystallization and
melting in dispersed emulsion droplets.
4.2 Potential of ultrasound in lemon juice industry
4.2.1 General consideration
The most frequent reason for the deterioration of lemon products is the development of microbial activity and this often results in moulding, fermentation and acidity. Spoilage of lemon juice is mostly caused by aciduric micro-organisms such as Leuconostoc species (Uelgen and Oezilgen, 1993). In addition there may be enzymatic transformations due to enzymes of the juice itself or those produced by micro-organisms. For the lemon juice product, thermal treatments are generally employed and should be sufficient to inactivate both microbes and enzymes, in particular pectinesterase (PE; pectinmethylesterase, pectase, pectinmethoxylase, pectin pectilhydrolase, EC 3.1.1.11) and polygalacturonase (PG; polygalacturonidase, pectinase, pectolase, and pectin-polygalacturonidase, EC 1.2.1.15) activities. Recent research has shown that PG is not the primary determinant of citrus fruit softening (Gross, 1991; Ketsa and Daengkanit, 1999). Therefore, PG itself may not be sufficient to induce cloud loss of the lemon juice. Besides, PE is the most heat-resistant cloud-destabilizing enzyme present in lemon juices. Inactivation of PE is generally used as
an indicator for the adequacy of pasteurization because it is known to be more heat resistant
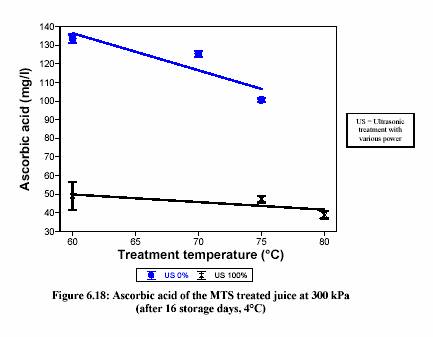
than the common micro-organisms. Ascorbic acid is the important nutrient in lemon juice,
but it undergoes degradation during pasteurization and storage. Inactivation rates of micro-organisms and enzymes and degradation rates of nutrients strongly depend on the pH and processing temperatures. A food processing system capable of adjusting the pH of juice by blending or substituting one citrus juice with others allows the optimum pasteurization pH and temperature for assuring maximum ascorbic acid retention while inactivating sufficiently micro-organisms and enzymes. There are numerous examples in the literature of optimization of the sterilization process for maximum nutrient retention by varying both pH and temperature (Uelgen and Oezilgen,
Although synthetic clouding agents have been used for years, recently several have been barred or their use restricted below a useful level. Therefore, from a legal perspective, a natural clouding would be preferred to synthetic agents. When citrus fruit are juiced, about 50% of their weight is left as waste peel, membranes, juice vesicles and seeds. One of the most promising products that can be made from this waste is a natural beverage clouding agent. The production of this new agent is also desirable because it helps to eliminate one source of pollution during citrus manufacturing (El-Shamei, Z. and El-Zoghbi, M.; 1994).
One problem in lemon juice pasteurization is that the high temperature results in flavour and aroma changes as well as losses in vitamins and volatile compounds. Inaddition, heat promotes browning reactions in the citrus juice. The magnitude of these changes increases with the increasing of time and temperature. The development of new technological procedures that would be more efficient for pasteurization would be a great advantage for lemon juice industry.
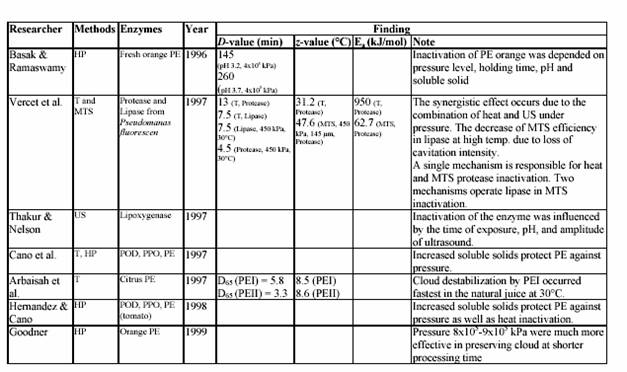
4.2.2 Conventional treatment for lemon pectinesterase inactivation
In order to prevent cloud loss, citrus juice is pasteurized at high temperature (90 C for 1 min) to inactivate PE (Eagermann and Rouse, 1976; Versteeg, 1979). Versteeg et al. (1979) demonstrated that citrus contains two isozymes of PE, which are heat labile at 70 C and a third isozyme, which is stable at temperatures up to 80 C. They also showed that cloud loss was due to improper inactivation of this heat stable isozyme. Since the inactivation temperature in this study was at 90 C, only the heat stable PE isozyme was of interest. Eagermann and Rouse (1976) examined the PE inactivation in juices extracted from different citrus varieties and found that the z-values ranged from 4.4-6.7 C depending on variety. PE inactivation was faster at low pH values than at higher values (Atkins and Rouse, 1953; Kew et al., 1957; Draetta et al., 1979). Nath and Ranganna (1977) showed that the processing time for PE inactivation increased as the pH increased. They demonstrated that juice stabilization could be achieved by processing 2D value at pH 3.6, while a 2.5D would be required to stabilize juice at pH 4.0. Moreover, the inactivation of PE was faster in high Brix than in single strength juices at 68.3 C (Atkins et al., 1956). However, at higher temperature (73.8 C and greater) erratic results occurred, indicating that PE inactivation was not faster in the higher concentration (Marshall, Marcy and Braddock, 1985). Braddock suggested that a decrease in the PE inactivation rate occurred indicated that the inactivation of PE may be protected at higher Brix. Increasing the enzyme concentration 4-fold showed that an S-shaped or sigmoidal curve resulted.
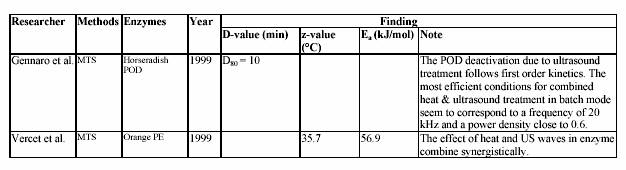
4.2.3 Potential of ultrasonic application in the lemon juice industry
Ultrasound processing in combination with lower heat process temperatures has many advantages. It can lead to better quality products, with improvements in taste, texture and appearance. It also could result in reduced energy requirements and therefore reduced cost. The application of ultrasound with heat will require the design of new types of processing equipment. The design of heat exchangers and heating system, which incorporate powerful ultrasonic transducers, will not be easy. The fact that heat can be applied after ultrasound treatment with useful effects is encouraging if one considers the engineering aspects. The compatibility of ultrasound processing with existing material handling and packaging systems is encouraging. Plant modification will be needed rather than complete equipment replacement: this has significance in terms of commercial realization and the demands on capital investment needed to put the technology to work. The likely applications are
difficult to predict at this stage but thermosonication and manothermosonication is particularly suited to pumpable liquids and liquid containing solids, which could be processed in a continuous flow arrangement with clean or aseptic filling. The prospect of applying ultrasound to foods already packed into containers is conceptually possible but the practical constraints need to be considered in details, particular in terms of ultrasound penetration. Although the possibility of deactivating enzymes or destroying microorganisms by ultrasound waves, alone or in combination with other physical treatments, has been widely used for laboratory applications in microbiology, immunology and enzymology, it is not true for industrial applications. The reasons of the non-development
on an industrial scale of this technique are numerous and in part the non-development is due to the lack of information needed for design and scale-up procedure (Mason, Lorimer and Bates, 1992).
Chapter 5
Materials and methods
5.1 Juice preparation
5.1.1 Preparation of lemon juice
For the experiment of discontinuous process, the 9 kg whole Spanish lemons were washed
at 4C and cut into little pieces by household mixer (Braun, Germany type 3210) at the
middle speed for 30 minutes. The slurry was blended with 1 M Tris (8 litre) containing 0.1
M in NaCl (pH 8.15) at 4C for 90 min. The solution was filtered through muslin. The
filtrate was added with ammonium sulphate (25% saturation) and centrifuged at 11600 x g
at 0C for 20 min to remove some pectin. The supernatant was added with 80% saturation
of ammonium sulphate and left overnight. The solution was centrifuged at 11600 x g at 0C
for 20 min. The supernatant was added with 10% water weight and centrifuged at 11600 x
g for 20 min. The pellet was redissolved with 900 ml Tris HCl (pH 7) containing 0.1 M in
NaCl and left overnight. The solution was then centrifuged at 12000 x g, 20 min. The
supernatant was precipitated with 80% saturation of ammonium sulphate. The solution was
centrifuged again at 12000 x g for 20 min. The pellet was diluted with Tris HCl (pH 7.0)
0.1 Min NaCl (~300 ml). The extract had approximately 64650 units of PE activity.
CHAPTER 5 MATERIALS AND METHODS
For the experiment of continuous process the lemons from Spain were purchased
from a local store (Berlin). 20 kg freshly chopped lemons were blended at 4C with 1 M
Tris (25 litre) containing 1 M NaCl. The slurry (pH > 8) was allowed to stand for 90 min,
before pressing through muslin. The filtrate was very viscous owing to the presence of
pectin. The filtrate was then added with ammonium sulphate (to 25% saturation) and
centrifuged (11500 x g, 15 min) to remove some of pectin. The solution was left overnight.
Protein and PE precipitation was made by addition of the ammonium sulphate (to 80%
saturation). After centrifugation (11500 x g, 15 min), the gelatinous pellet was redissolved
in 2 litre of Tris HCl (pH 7.0) with 0.1 M in NaCl and left overnight to allow precipitation
of pectates produced by PE action. After centrifugation (11500 x g, 15 min), the
supernatant was stored at -20C until required. The yield of PE was 104500 Units. The
freeze extracted samples containing PE enzyme were redissolved in distilled water, pH 7.0
and heated at 70C for 5 min to obtain only heat-stable portion of enzyme. Then the activity
of enzyme solution was measured at various temperatures and duration.
For the experiment in the pilot plant the 250 kg of Spanish lemons were squeezed
by a household squeezer (Braun, Germany) and filtered through muslin in order to have
clear juice. Juice was maintained at temperature approximately 4C by subjecting the ice
bags to the container. The juice has initial pH 2.45 and light green-yellow colour before
applying to the process system.
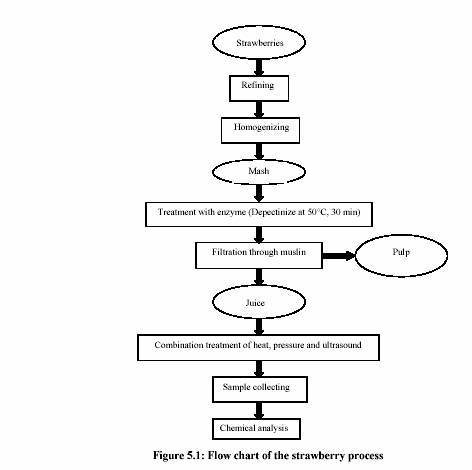
5.1.2 Preparation of strawberry juice
Frozen strawberries (60 kg) were obtained from the strawberry juice company (TYC,
Poland). The strawberries for the processing were selected to be mature and disease free.
Strawberry puree, obtained by homogenization using a blender (Stephan MicRoCut; MC H
20 k; STEPHAN und Shne Co. Hameln, Germany), was depectinized by the enzyme
solution (Pectinex Ultra SP-L; Novo Nordisk Ferment Ltd., Switzerland) (Dosis 0.5%; at
50C for 30 min) in order to achieve maximum juice yield. The juice was filtered through
muslin to obtain clear juice before applying to the MTS treatment system. The treatment
process was summarized by the flow chart in Figure 5.1.
5.1.3 Preparation of tomato juice
14 kg fresh tomatoes (ALP Co., Turkey) were purchased from a local shop. Fresh tomatoes
were mixed with cold 1 M NaCl solution (4C) in the proportion 1:1 in the household blender (Braun, type 3210-645, Germany) for 30 sec with maximum speed. The slurry was stored in a large container with ice bags (~ 4C). The solution was centrifuged at 3200 x g for 5 min (Minifuge RF; Heraeus Sepatech, Germany). The supernatant was dialyzed through the dialyze tube (pore diameter 0.0025-0.005 m, cut off 10000-20000, Roth, Horn Karlsruhe) and left in the distilled water (4C; solution : water = 1:15) for 3 hours. The conductivity of the solution after the dialyzation was 5.1 mS/cm. The dialyzed solution was kept in polyethylene bags and stored at -20C.
5.2 Equipment and experiment set-up
5.2.1 Discontinuous unit
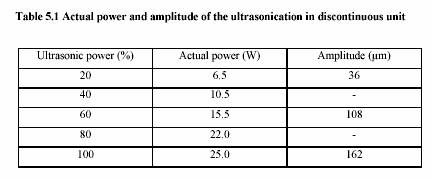
A submerged ultrasonic horn for 100 l - 50 ml, with a tip diameter of 3 mm and fixed frequency 20 kHz, was used. A generator (Bandelin Electronic, Berlin) converts 50 Hz electrical energy into 20 kHz. A transducer containing the piezo-electronic element enabled the conversion of 20 kHz electrical energy to vibrating mechanical energy of the same frequency.
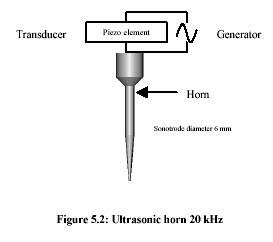
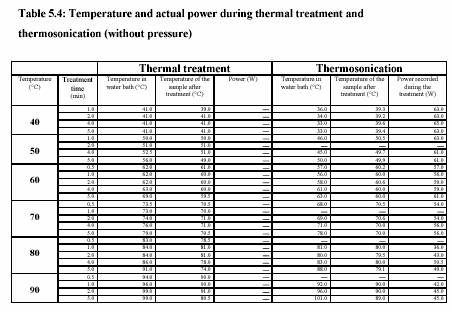
Experiment performed in water bath adjusting temperature to 40C-90C. Two thermometers were placed either in solution and water bath to observe the temperature profile. Time was started measuring as soon as the temperature of the solution reached the water bath temperature.
Continuous unit
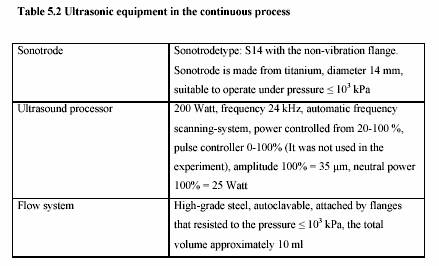
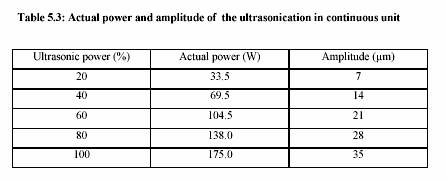
The ultrasonic treatment was performed in the continuous ultrasonic system (Dr. Hielscher,
Berlin, Germany). Table 5.2 presents the technical data of the ultrasonic equipment.
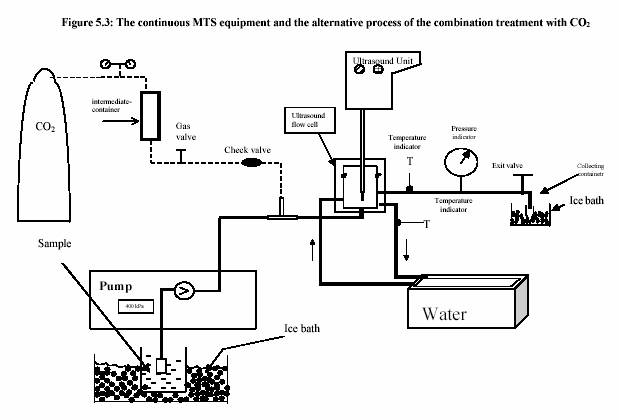
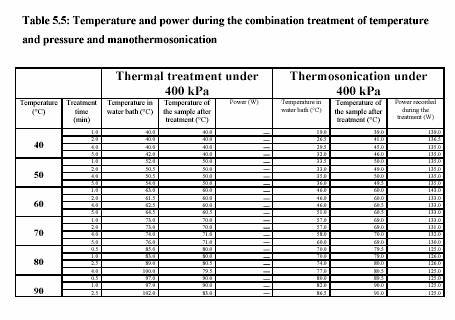
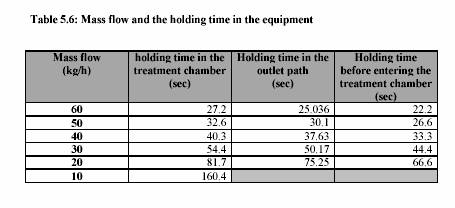
The piston pump (Knauer, HPLC pump, type 6400, Berlin) drew the sample through the system. The flow rate was adjusted to keep the flow continuous. For the operation with pressure 400 kPa, the outlet valve was controlled to maintain the constant pressure. Figure 5.3 presents the equipment set up. The combination treatment of ultrasound and CO was also carried out in the same equipments. The pressure was considered to be the pressure of CO . The temperature of the sample during the experiment was constantly controlled by the water bath. The temperature profiles were also recorded during the experiment. Table 5.4 and Table 5.5 are the experiment conditions, which show the treatment temperature, pressure, time for the treatment with and without ultrasound.
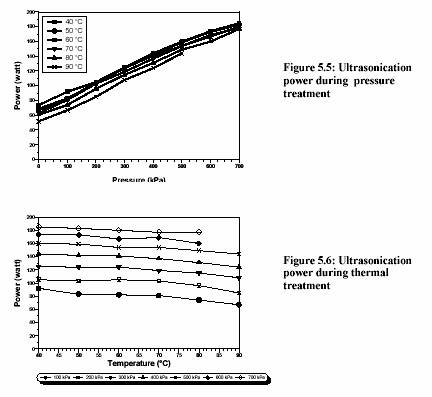
The ultrasound energy is also depended on treatment temperature and pressure (Figure 5.5 and Figure 5.6). Increased pressure dramatically increases the ultrasound power (Figure 5.5). Contrarily, the increased temperature slightly decreases the ultrasound power (Figure 5.6).
Sonotrode
Power: 200 W
Ultrasonic power: 20% - 100%
Frequency: 24 kHz
Dimension: 190 x 200 x 90 mm (Length x Width x Height)
Weight : 2.3 kg
Generator
Electricity: 230 V, 8 A, 50-60 Hz
115 V, 16 A, 50-60 Hz
Thermosonication
Heat treatments were performed between 40C - 80C in the treatment chamber surrounded by the cooling jacket. The flow rate was controlled by the HPLC. The holding time was converted from the HPLC flow rate chart. The ultrasound power was adjusted to 100% to perform the maximum reaction.
Manothermosonication
Manothermosonication was performed in the treatment chamber surrounded by the cooling
jacket. The operating temperatures were set from 40C to 80C. Inside the vessel (10 ml), the treatment chamber was fitted with the sonotrode model UP200S from Dr. Hielscher GmbH, Germany. The ultrasonic horn irradiates at a fixed frequency of 20 kHz. Pump HPLC allowed the enzyme solution to the treatment chamber at the adjusted flow rate. The pressure (100 - 300 kPa) was manually controlled by the outlet valve.
Pilot plant unit
The experiments were performed in a continuous system combining of temperature, pressure and ultrasound (Zenker et al., 1999).
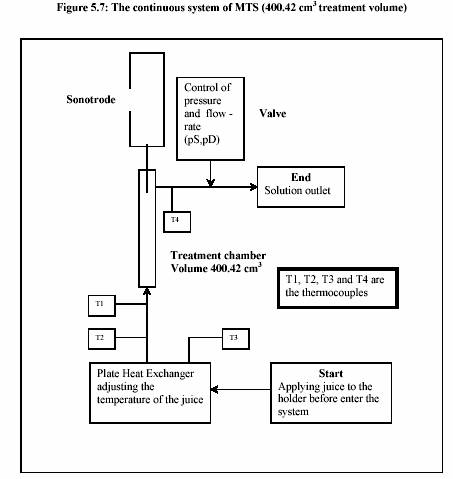
The sonotrode (UIP 1000 Ultrasound-Industrial processor 1000 W, Dr. Hielscher Co., Ltd., Berlin) was manipulated in the experiment. The technical data are as follows:
Sonotrode
Power: 1000 W
Usual power: 20% - 100%
Frequency: 20kHz
Maximum amplitude: 35 45 m
Dimension: 112 x 71 x 440 mm (Length x Width x Height)
Flange-diameter: 100 mm
Generator
Electricity: 230 V, 8 A, 50-60 Hz
115 V, 16 A, 50-60 Hz
19'-System: 363 x 365 x 153 mm (Length x Width x Height) of the case.
Zenker et al. (1999) also stated that the ultrasonic power was slightly decreased from 700 W to 650 W when the temperature of the medium increased from 30C to 80C at 300 kPa.
Juice was held in the holding tank before entering the system by the controlling valve. The closed valve allows the system initially operated with the distilled water in order to adjust the required temperature, pressure and flow rate. After the system reached the equilibrium, the juice was necessary to be subjected to the system by the immediate opening of the valve; otherwise a huge pressure drop may occur.
The juice was heated up to the required temperature by passing through the heat exchanger. The temperature was shown at the outlet of the heat exchanger (T2). The flow rate was adjusted through out the experiment in order to keep the pressure constant, which functioned by opening and closing the ventilation valve at the outlet. In this experiment, the pressure was stable at 300 kPa.
Four thermometers were installed at the inlet (T3) and the outlet (T2) of the heat exchanger, inlet (T1) of the treatment chamber and at the outlet (T4). The temperatures were measured through out the process. The outlet temperature (T4) was taken into account as the control temperature.
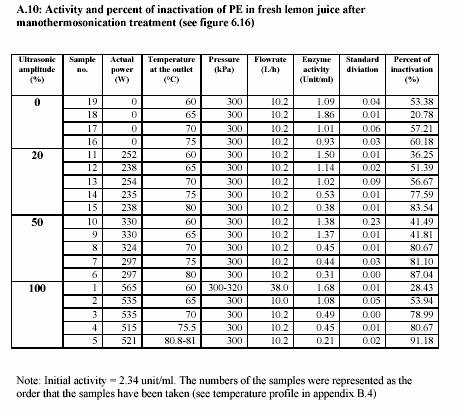
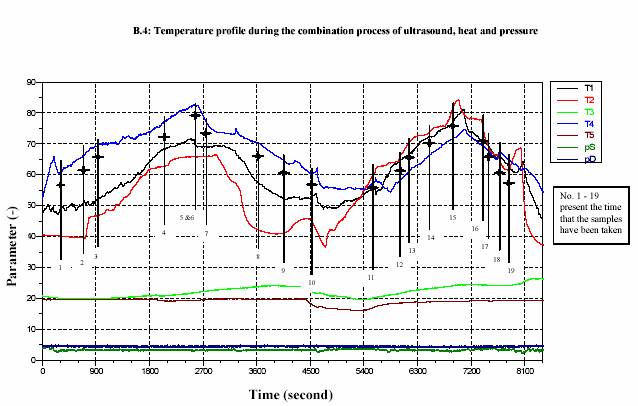
After the heating section, the temperature of the juice slightly decreased due to the energy loss before entering the adjacent area of the sonotrode. The advantage of the decreasing of the temperature is to overcome the desired value of temperature after sonification. The temperature was then again significantly increased during the sonification. The process pressure was simultaneously controlled by the ventilation valve (at the outlet) and the hydraulic pump. Sizes of the pumps also affected the pressure control. Large pump could give more stable value of high pressure rather than small pump. In contrary, the small pump controlled low pressure more successfully than large pump. The experiments were operated at different temperatures and different powers of
sonification 0%, 20%, 50% and 100%. These different powers of sonification gave different value of circuit power (Watt) (see Appendix A.10 for the lemon juice and Appendix A.13 for the strawberry juice). The actual values of power were recorded during the treatment. The pressure was constantly maintained at 300 kPa and most of the experiments were run at approximately 10 L/h (Appendix A.10 for the lemon juice and Appendix A.13 for the strawberry juice).

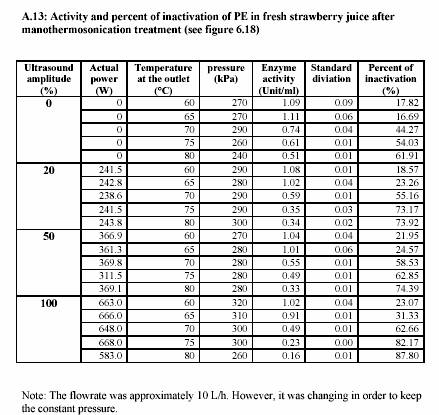
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 6166
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2026 . All rights reserved